Case report
Ukrainian Neurosurgical Journal. 2024;30(3):56-60
https://doi.org/10.25305/unj.307877
Vitaliy Y. Molotkovets 1,2, Oleksii S. Nekhlopochyn 3, Myroslava O. Marushchenko 1
1 Department of Neurosurgery, Bogomolets National Medical University, Kyiv, Ukraine
2 Extracerebral Tumor Department, Romodanov Neurosurgery Institute, Kyiv, Ukraine
3 Spine Neurosurgery Department, Romodanov Neurosurgery Institute, Kyiv, Ukraine
Received: 05 July 2024
Accepted: 31 July 2024
Address for correspondence:
Vitaliy Y. Molotkovets, Extracerebral Tumor Department, Romodanov Neurosurgery Institute, 32 Platona Maiborody st., Kyiv, 04050, Ukraine, e-mail: molotkovets@gmail.com
Spinal meningiomas are rare, predominantly benign tumors that exhibit slow growth and typically have a non-invasive pattern of development. They originate from arachnoid cells and fibroblasts of the dura mater. Despite their benign nature, some meningiomas can exhibit intra-extradural extension, complicating both diagnosis and treatment. This article presents a clinical case involving a patient with an intra-extradural spinal meningioma. Despite radiological imaging suggesting a neurinoma, the final diagnosis confirmed a meningioma.
Case Report: A female patient underwent surgical tumor resection through a posterolateral approach with laminectomy and facetectomy at the C4-C5 vertebral levels. The tumor, extending through the intervertebral foramen, was completely resected along with the affected nerve root. Histological examination verified a Grade 2 meningioma.
Discussion: Despite advancements in neuroimaging and surgical techniques, intraoperative findings can be unpredictable, necessitating an adaptive approach to tumor resection. The article emphasizes the importance of adequate preoperative planning and the use of intraoperative neurophysiological monitoring to reduce the risk of complications and improve treatment outcomes.
Conclusions: The primary treatment for spinal meningiomas is surgical. For dorsal and lateral localizations, total resection with the involved dura mater (Simpson Grade I) is optimal. For ventral localizations, tumor resection with coagulation of the dural attachment site (Simpson Grade II) is preferred. Preoperative and intraoperative use of electrophysiological methods is recommended to assess the functional status of neural structures. Intra-extradural localization of meningiomas is rare and presents significant challenges in preoperative diagnosis, requiring specific skills for effective removal.
Keywords: meningioma; spinal tumor; intradural extramedullary location; neurosurgery
Introduction
Meningiomas are slowly progressing benign neoplasms. They originate from arachnoid cells and fibroblasts of the dura mater (DM) [1]. They are characterized mainly by a non-invasive growth pattern but can extend to adjacent tissues. Meningiomas account for 25-46% of primary extramedullary spinal tumors [2,3] and 1.2-12.0% of all meningiomas of the central nervous system [3, 4]. In terms of their location relative to the spinal cord, they can be lateral (55%), ventral (29%), dorsal (13%), or “dumbbell”-shaped (3%) [4], and in relation to the DM, they can be intradural and extradural. In some cases, only extradural localization is observed [5], which complicates radiological assessment during preoperative diagnosis. Arachnoid tissue migration with islet aberrations in the case of extracranial meningiomas and distant sites (e.g., the nose or skin) is assumed [6, 7]. This mechanism of spread may occur with isolated extradural spinal meningiomas.
They occur more frequently in women aged 50-80, which is associated with hormone receptor expression [8, 9]. Meningiomas are the second most common benign extramedullary tumor after schwannomas in women aged 40-70 [10]. About 9% of spinal meningiomas are asymptomatic [11]. Depending on the location and size of the tumor, the clinical picture may manifest as pain, motor and sensory disorders, ataxia, and pelvic organ dysfunction [12]. Pain occurs in 42-87% of cases and may be either local or irradiated [13]. The clinical picture in extradural spread does not significantly differ from that of an intradural tumor.
Magnetic resonance imaging (MRI) with intravenous contrast enhancement is the diagnostic standard. Since extradural localization is relatively rare for meningiomas, careful evaluation of the radiological picture is required. For example, foraminal extension is grounds for suspecting schwannoma or neurofibroma [12]. In the presence of young patients or multiple lesions, a genetic disease (neurofibromatosis type 2) is possible [14]. Meningiomas should be primarily differentiated from schwannomas, metastatic tumors, lymphomas, and tuberculomas. Preoperative differentiation and intraoperative histological examination allow the optimal surgical approach to be determined [15].
The use of the modified McCormick scale is advisable to assess functional impairments in spinal neoplasms (I - neurologically intact patients who move normally, with minimally expressed sensory disorders possible, II - mild motor or sensory deficits, functionally independent patients, III - independent of external assistance, IV - gross motor or sensory deficits, functional limitations, patients dependent on external assistance, V - paraplegia or tetraplegia) [16].
Clinical Case
Patient K., a 36-year-old military servicewoman, presented with complaints of pain in the cervical spine, slight muscle weakness in the left arm, dizziness, and impaired sensetion in the left arm. Neurological examination revealed a slight decrease in muscle strength (4 points) during shoulder abduction, characteristic of C5 root involvement.
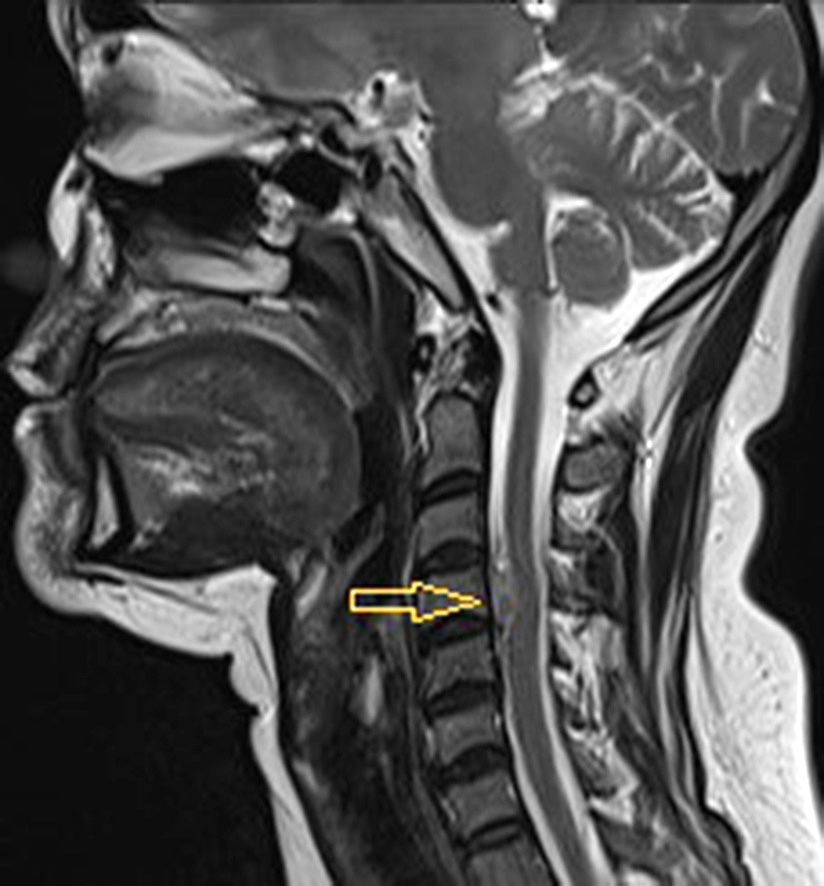
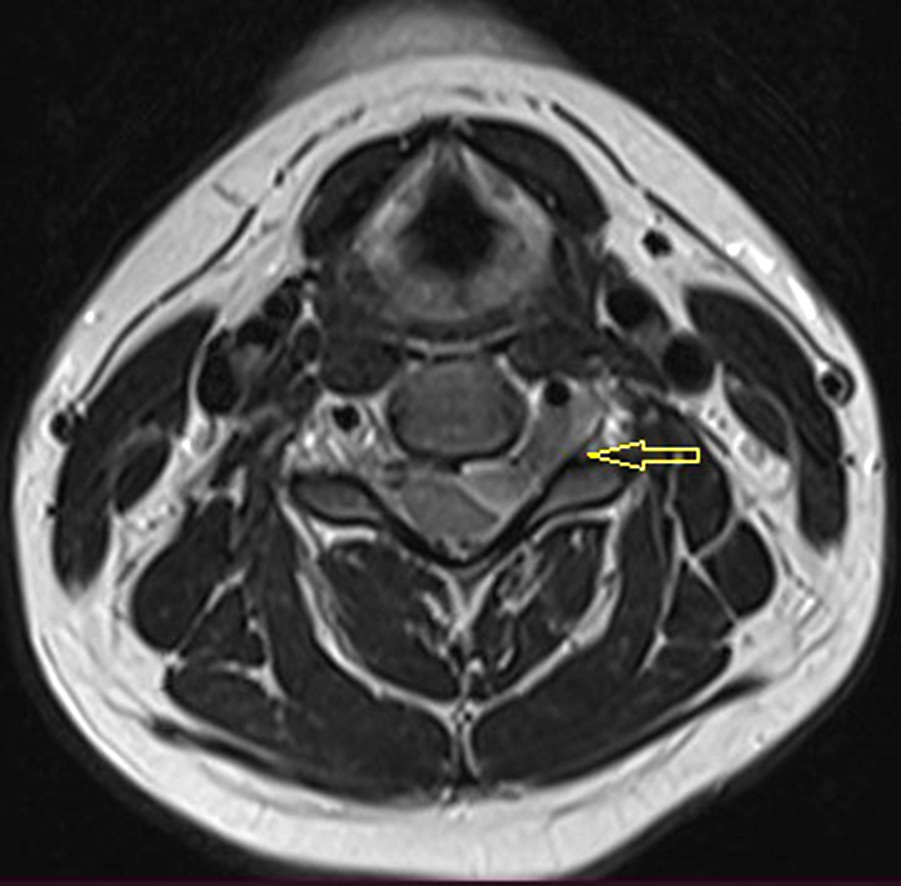
MRI with contrast agent showed an intra-extravertebral tumor extending through the intervertebral foramen. The radiological picture most closely resembled a schwannoma (see Fig. 1).


Fig. 1. MRI of the cervical spine with contrast in T2-mode. The arrow indicates an intra-extravertebral tumor extending through the intervertebral foramen. Tumor size: 13.0×22.0×8.5 mm
Surgery was performed to remove the tumor (Simpson II). Posterolateral approach was chosen for access to the tumor. C4 and C5 vertebral arches and articular processes together with the joint were identified through a midline incision. A laminectomy at the C4-C5 vertebrae and a left C4-C5 facetectomy were performed. The expanded intervertebral foramen and the dural cuff, showing signs of significant tension, were noteworthy. The intradural portion of the tumor was removed in the first stage. The nerve root was dissected and separated from the tumor tissue. The dural cuff was incised, and the portion of the tumor located in the intervertebral foramen and extending extravertebrally was removed. Within the dural cuff, the nerve root was structurally destroyed, making it impossible to preserve the nerve. After removing the tumor along with the affected nerve root, the DM was coagulated at the tumor growth site. Hermetic suture of the DM was performed at the site of the nerve root opening and tumor spread.
The postoperative period was uneventful. Neurologically, the patient did not experience any deterioration. She was referred for further rehabilitation treatment. Subsequently, the patient returned to her duties.
A meningioma (Grade 2) was verified by pathohistological examination.
MRI performed six months after surgery showed no signs of pathological accumulation of contrast agent which could indicate tumor recurrence (Fig. 2).
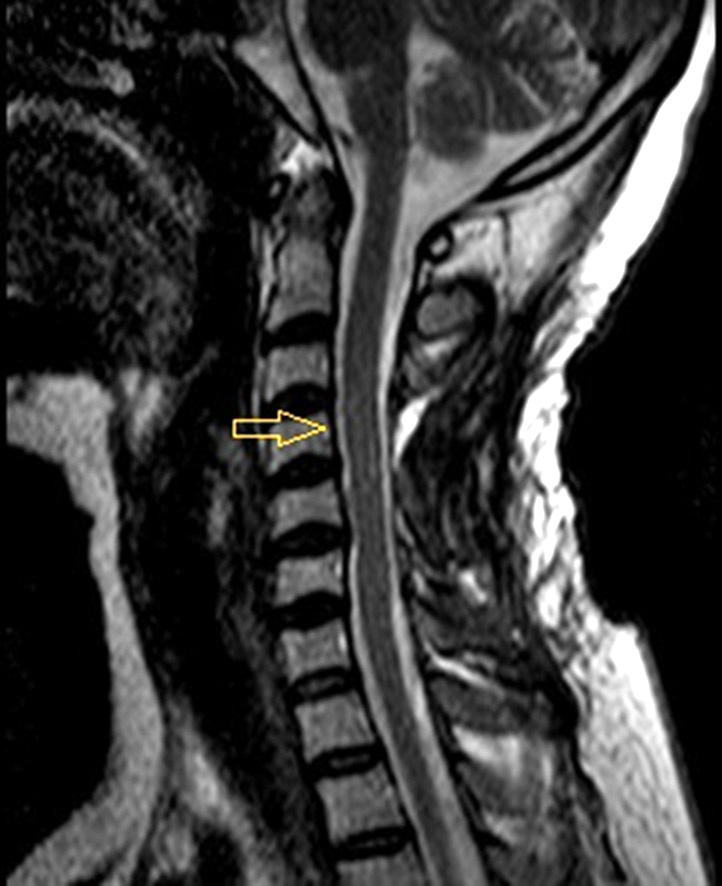
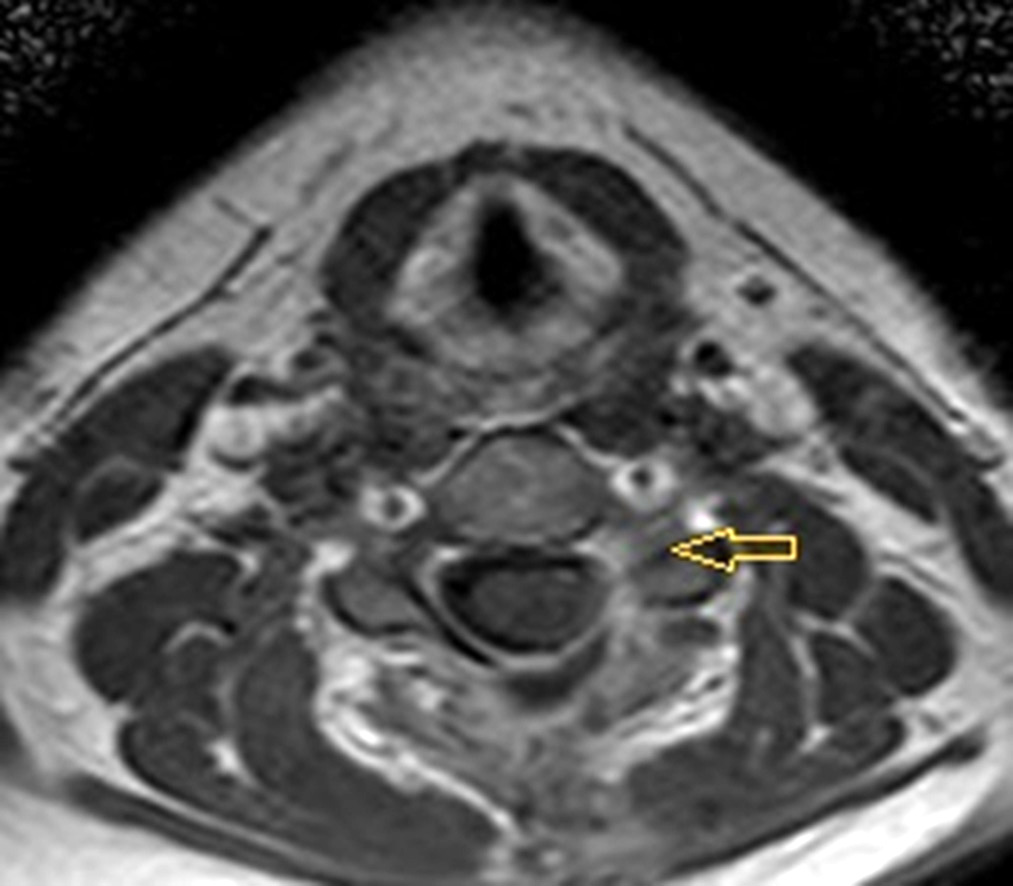
Fig. 2. MRI of the cervical spine with contrast, T2 mode. Postoperative follow-up six months after meningioma removal. Simpson II
Discussion
The primary treatment for spinal meningiomas is surgical, which is the most effective method for reducing recurrence rates (3-15%) after removal [10,17]. The generally accepted surgical strategy is radical removal of CNS meningiomas (Simpson I), as subtotal resection is a recurrence factor. Proven additional recurrence factors of spinal meningiomas include young age (<18 years), cervical spine location, extensive “dural tails,” and male gender [18-20]. Given the complexity of DM plasty and the risk of damage to neural structures and disruption of DM integrity, spinal meningiomas are predominantly removed using the Simpson II method. However, there is no significant increase in recurrence rates as with cranial meningiomas. The recurrence rate for Simpson II removal is 1-8% [4, 17, 21]. The type of removal (Simpson I or Simpson II) is debated in the case of spinal meningiomas due to the low risk of recurrence and the increase in complications. No significant difference in survival has been established between different removal types (Simpson I or Simpson II) [4].
In most cases, total removal of meningiomas is achieved [22]. To some extent, the radicality of removal depends on the location. Dorsal and dorsolateral meningiomas are more often successfully removed with DM resection and reconstruction [4]. When removal of the growth area is impossible, dissection and splitting of DM leaves are recommended to increase radicality and maintain sheath integrity [23]. It has been established that one-third of spinal meningiomas invade the DM, specifically spreading between the inner and outer leaves, and 47% have “dural tails,” as confirmed by pathohistological studies [24]. In the study by K. Kobayashi et al., of 116 spinal meningiomas, 3 had a dumbbell shape with transforaminal, extravertebral spread. After Simpson II removal, recurrences were observed in these three cases [4].
Considering the need to preserve neural structures during radical tumor removal, it is recommended to use intraoperative neurophysiological monitoring, including transcranial motor evoked potentials, and during preoperative diagnosis, to conduct electroneuromyography with an assessment of the degree of conduction impairment in the proximal sections of the nerve and innervation [25].
The main surgical treatment methods are [26]:
The choice of surgical method depends on many factors, such as tumor aggressiveness and complex spreading patterns (e.g., anterior spinal cord localization in the thoracic spine or anterolateral localization relative to the upper cervical spine, which may compress the vertebral artery [27]).
Optimal surgical access to ventral extra-intravertebral meningiomas of the cervical spine is challenging [28]. Both anterior approaches with corpectomy and corporodesis and posterior approaches with laminectomy and partial unilateral facetectomy are equally effective [28]. The main principle for choosing an approach to ventral extra-intravertebral meningiomas of the cervical spine is the level of involvement: for upper cervical localization, a posterior approach is preferred, and for lower cervical localization, an anterior approach is preferred.
Postoperative mortality for spinal meningiomas is generally low, ranging from 0 to 4.7%, according to various authors [29,30].
Radiation therapy can be applied in cases of high-grade malignancy or recurrence [31].
The presented clinical case shows that despite significant improvements in neuroimaging methods and adequate preoperative planning of the intervention volume, intraoperative findings can sometimes be unpredictable. The presence of classic, almost pathognomonic signs of schwannoma (dumbbell shape accompanied by anatomical enlargement of the intervertebral foramen) is the basis for even experienced surgeons to choose a certain volume of intervention. The detection of a meningioma localized intraextracanalically requires significantly more effort for its adequate removal, minimizing the risks of neurological consequences and general surgical complications, such as cerebrospinal fluid leakage or the formation of cerebrospinal fluid cysts. Awareness of such situations is of important practical significance both for the radical removal of the tumor and for the necessary handling of the DM, which is critical to reducing the risk of recurrence.
Conclusions
The main treatment method for spinal meningiomas is surgical intervention. For dorsal and lateral localization, the optimal approach is total removal along with the involved DM (Simpson I). In the case of ventral localization, preference is given to tumor removal and coagulation of the site of derivative growth (Simpson II).
Electrophysiological methods are recommended during the preoperative and intraoperative periods to assess the functional state of neural structures.
Intra-extracanal localization of meningiomas is rare. It can significantly complicate preoperative diagnosis and may require specialized skills for the removal of such meningioma.
Disclosure
Conflict of Interest
The authors declare no conflicts of interest.
Informed consent
Informed consent for data disclosure was obtained from the patient.
References