Original article
Ukrainian Neurosurgical Journal. 2025;31(1):23-33
https://doi.org/10.25305/unj.315024
1 Spine Surgery Department, Romodanov Neurosurgery Institute, Kyiv, Ukraine
2 Restorative Neurosurgery Department, Romodanov Neurosurgery Institute, Kyiv, Ukraine
Received: 12 November 2024
Accepted: 16 December 2024
Address for correspondence:
Oleksii S. Nekhlopochyn, Spine Surgery Department, Romodanov Neurosurgery Institute, 32 Platona Maiborody st., Kyiv, 04050, Ukraine, e-mail: AlexeyNS@gmail.com
Instability from traumatic spinal injuries is a major indication for urgent stabilizing surgery to prevent adverse consequences of pathological mobility in the injured spinal segment. However, the staged evacuation of injured individuals from active combat zones and the need for urgent life-saving interventions often delay stabilization, leading to an increase in cases of delayed stabilization for unstable spinal injuries. Clinical analysis of such cases has revealed specific features that are underrepresented in the literature. One of these is post-traumatic aseptic necrosis in unstable injuries, which differs in several respects from Kummel disease and requires detailed characterization.
Objective: To characterize and conduct a preliminary analysis of clinical cases of post-traumatic aseptic necrosis of the vertebral body in unstable thoracolumbar spinal injuries.
Materials and Methods: A retrospective analysis was conducted on a patient database of those receiving inpatient treatment at the Romodanov Neurosurgery Institute of National Academy of Medical Sciences of Ukraine, between 2021 and 2024, as well as patients consulted in Kyiv healthcare institutions, either in person or via telemedicine. The primary inclusion criterion was a verified unstable traumatic injury of the thoracolumbar spine, where surgical stabilization was not performed within two weeks post-injury.
Results: Nineteen cases of delayed stabilization for unstable thoracolumbar injuries were identified through medical documentation and imaging data. Six (31.6%) of these cases exhibited signs of aseptic post-traumatic vertebral necrosis. Clinical examples of patients with and without developed spondylonecrosis are presented. The groups were compared based on demographic and trauma-specific characteristics; however, statistically significant predictors for spondylonecrosis development were not identified. Additionally, the presence of chronic septic processes was not found to contribute to this phenomenon. Follow-up data indicated that delayed stabilization contributed to halting bone tissue lysis. Relevant literature on spondylonecrosis and a cascade of pathological processes potentially leading to this condition are discussed.
Conclusions: This publication is among the first to describe post-traumatic aseptic vertebral body necrosis in unstable thoracolumbar spinal injuries. The data and proposed pathogenic mechanisms emphasize the importance of early stabilization for unstable injuries from both neurological and orthopedic perspectives.
Keywords: thoracolumbar spine; traumatic injury; instability; aseptic vertebral body necrosis; delayed stabilization
Introduction
The stability of traumatic spinal injury is one of the fundamental criteria determining not only the general strategy and individualized tactics of patient management but also, in some cases, the prognosis for the restoration of functional activity. According to the classic concept proposed by M. Panjabi et al., spinal stability refers to the spine's ability, under physiological loads, to maintain intervertebral relationships that prevent both initial injury and subsequent irritation of the spinal cord or nerve roots, as well as the development of deformity or pain [1].
Spinal stability relies on the interaction of three systems: the passive system (comprising bony structures, intervertebral discs, and ligamentous apparatus, which mechanically limit mobility), the active system (the muscular corset, which provides dynamic stabilization), and the control system (the nervous system, which regulates and coordinates activity to maintain stability). Disruption of one (as in neurologically uncomplicated trauma) or several (in cases with neurological impairments) of these systems leads to functional spinal instability [2].
The zones of functional activity within the spinal motion segment (SMS) are an objective criterion in classical spinal biomechanics that define a specific injury. The neutral zone is characterized by the range of motion where the SMS experiences minimal resistance from passive structures, with minimal load on stabilizing elements. The elastic zone lies beyond the neutral zone, where resistance from passive structures increases, and the SMS begins to encounter significant resistance to limit overmobility. The overload zone is defined by the failure of compensatory mechanisms, leading to primary (describing the immediate trauma mechanism) or secondary (describing an already injured SMS) damage to both osteo-ligamentous and neural structures [3, 4].
This brief description of the mechanics of an injured SMS indicates that the concept of stability or instability is, to some extent, relative. Most often, an injured SMS, in the absence of mechanical load, remains in a state of relative stability. The transition from an expanded neutral zone through a shortened elastic zone to the overload zone depends on the intensity of the applied force [5]. In some cases, the mechanical integrity of the injured SMS is sufficient to maintain an upright posture but inadequate to perform everyday functional activities [6, 7].
The term "conditionally unstable injuries," previously widely used to describe such conditions, has been removed from clinical practice, complicating the description of functional impairments in these patients [2].
Spinal instability is a primary indication for stabilization surgery, aimed at mitigating the adverse effects of instability. These include preventing the onset or progression of neurological deficits, creating optimal conditions for resolving neurological dysfunction when present due to trauma, halting the progression of deformities through structural support provided by implants, facilitating consolidation, reducing pain intensity, and consequently improving the quality of life for patients while enabling more effective rehabilitation [8]. Evidently, the timing of surgical correction for unstable spinal injuries is largely determined by the risk of neurological deterioration, and to a lesser extent, orthopedic complications [9]. Notably, 15% of neurological deficits in spinal trauma cases do not manifest immediately but develop later, likely due to instability [10]. The progression of the deformity increases the extent of intraoperative correction required, which in turn complicates and prolongs the surgical procedure.
The escalation of the Russian Federation's aggression against Ukraine into active hostilities in 2022 led to a significant rise in injuries among both military personnel and civilians. This increase included a notable rise in spinal trauma cases, predominantly closed injuries, reflecting the nature of combat operations. A similar trend was observed in other conflicts, such as in Iraq and Afghanistan, where the primary causes of injuries were blast waves and mechanical forces [11, 12].
Modern protective gear, such as body armor and helmets, significantly reduces the risk of penetrating wounds but does not fully protect against closed injuries caused by blast waves, severe mechanical impacts, or falls [13]. The use of high-powered ammunition in contemporary armed conflicts generates shock waves that can cause severe spinal injuries upon impact. Additionally, these blast waves are often accompanied by extensive structural collapse, resulting in secondary injuries from falling debris [14].
The significant strain on the healthcare system, the staged process of medical evacuation, and the large number of polytrauma patients have led to an increase in delayed stabilization of unstable spinal injuries. This approach primarily applies to neurologically intact patients, where dynamic observation minimizes the risk of dysfunction, or to patients presenting with complete spinal cord injury. Such a strategy is a necessity under wartime conditions and is not typically employed in peacetime. The analysis of clinical cases involving delayed stabilization of unstable spinal injuries has revealed aspects that are underrepresented in the literature. One of these is post-traumatic aseptic necrosis of an unstable injury, which is the focus of this article. The only previously documented form of post-traumatic aseptic necrosis of the vertebral body (ANVB) is Kummell’s disease, described by the German surgeon in 1891 [15]. However, the cases we have documented demonstrate fundamental (in totality of features) differences from Kummell’s disease requiring a detailed description and analysis.
Objective: To characterize and conduct a preliminary analysis of clinical cases of aseptic post-traumatic vertebral body necrosis in unstable thoracolumbar spinal injuries.
Materials and Methods
Study Design: retrospective observational study.
A retrospective analysis was conducted on a patient database from the Romodanov Institute of Neurosurgery, National Academy of Medical Sciences of Ukraine, covering the period from 2021 to 2024. This included patients who received inpatient care or were consulted either in person or via telemedicine at healthcare institutions in Kyiv, to identify cases of the specified pathology.
Inclusion Criteria
- Presence of a verified unstable traumatic injury of the thoracolumbar spine, with no surgical correction performed within 2 weeks post-injury.
- Availability of high-quality computed tomography (CT) scans taken within the first 3 days post-injury and follow-up scans obtained 2 weeks or later.
Exclusion Criteria
- Presence of penetrating spinal injuries and/or bullet or shrapnel injuries in the paravertebral region, regardless of fracture zone.
- Clinical and/or laboratory signs of septic spondylitis/spondylodiscitis.
- History of spinal trauma of any severity and/or prior spinal surgeries.
The analyzed variables included gender, age, and the mechanism of injury. Neurological deficits were assessed using the American Spinal Injury Association (ASIA) criteria [16]. The injury pattern was evaluated using the AOSpine Thoracolumbar Spine Injury Classification System (TLSICS) [17], while injury severity was graded according to the Thoracolumbar Injury Classification and Severity Score (TLICS) [18]. Imaging data, including spondylography, magnetic resonance imaging (MRI), and spiral computed tomography (CT), were analyzed using the RadiAnt DICOM Viewer software (Medixant, Poland, Version 2023.1, License No. 1860F047).
Statistical Analysis
Data processing was performed using R (version 4.0.5, R Foundation for Statistical Computing) in the RStudio development environment (version 1.4.1106).
Results
The available medical records were analyzed, as well as the results of examinations of the affected individuals provided for remote consultation. Nineteen clinical cases meeting the inclusion criteria were identified (Table 1). Post-traumatic ANVB phenomena were verified in 6 (31.58%) of the affected individuals. Several clinical cases are presented as examples.
Table 1. Brief Characteristics of Patients
|
Parameter |
Value |
|
Sex: |
|
|
male |
12 (63,16%) |
|
female |
7 (36,84%) |
|
Age, years: |
|
|
median (95% confidence interval) |
40 (24‒48) |
|
range |
18–61 |
|
Injury circumstances: |
|
|
road traffic accident |
5 (26,32%) |
|
fall from height |
8 (42,11%) |
|
fall on a flat surface |
6 (31,58%) |
|
Injury level: |
|
|
thoracic |
5 (26,32%) |
|
thoracolumbar junction |
9 (47,37%) |
|
lumbar |
5 (26,32%) |
|
Type of injury (AOSpine): |
|
|
B2 |
9 (47,37%) |
|
B3 |
1 (5,26%) |
|
C |
9 (47,37%) |
|
Neurological deficit (ASIA): |
|
|
A |
12 (63,16%) |
|
B |
3 (15,79%) |
|
E |
4 (21,05%) |
|
TLICS, points |
|
|
5 |
3 (15,79%) |
|
6 |
2 (10,53%) |
|
7 |
3 (15,79%) |
|
8 |
9 (47,37%) |
|
9 |
3 (15,79%) |
Clinical case No.1
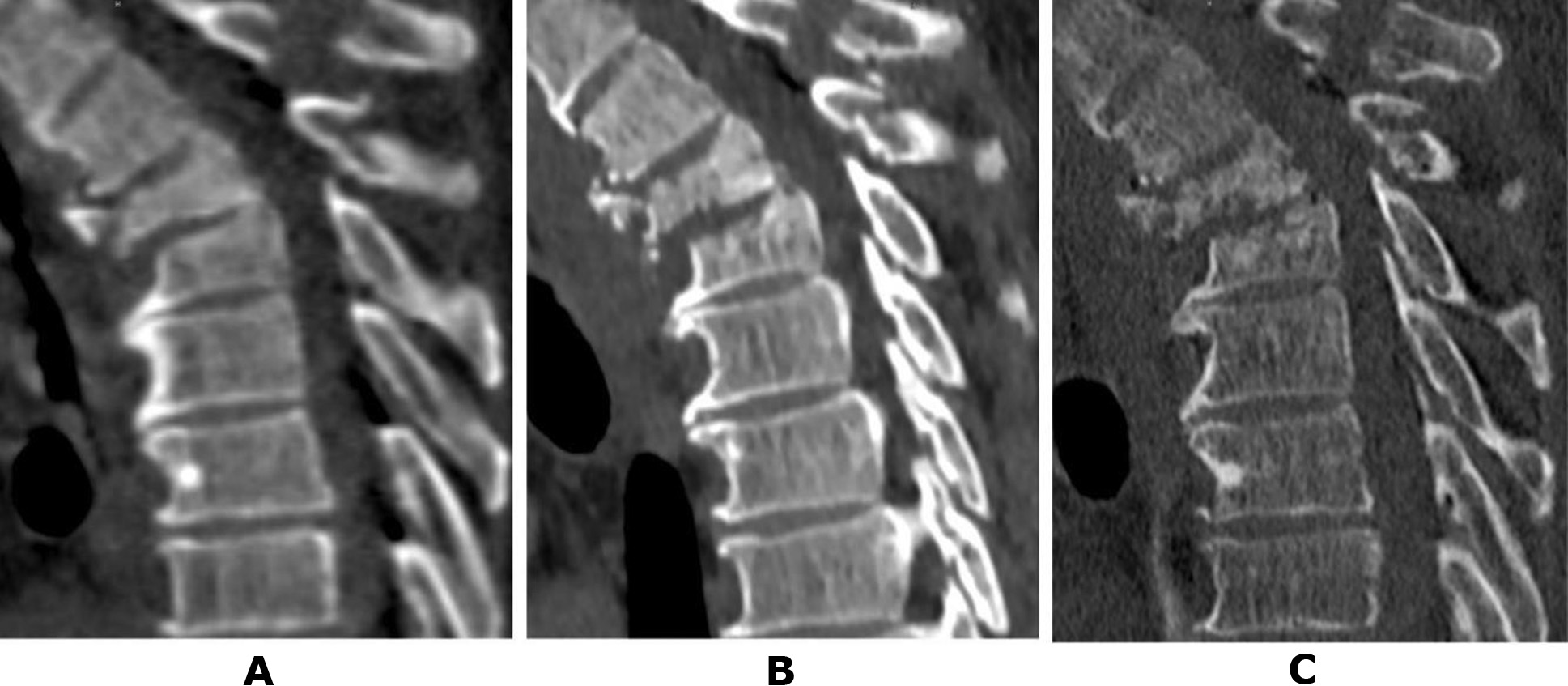
Patient N., a 50-year-old military serviceman, sustained injuries as a result of a road traffic accident (RTA) while driving. During initial hospitalisation at a military hospital, CT scans of the cervical, thoracic, and lumbar spine were performed. Diagnosed injuries included an incomplete burst fracture of the vertebral body and a comminuted fracture of the vertebral arch at Th3 (type B2), a compression fracture of Th4 (type A1), and a fracture of the spinous process of Th2 (Fig. 1A). The neurological status of the patient corresponded to ASIA E. Given the preserved neurological functions and certain technical challenges associated with performing stabilisation surgery, the patient was evacuated in stages and subsequently admitted to the Romodanov Institute of Neurosurgery, NAMS of Ukraine. CT scans conducted at each hospital allowed monitoring of the dynamic changes in the bony structures at the injury site. Two weeks post-injury (Fig. 1B), initial signs of lysis in the anterior sections of the Th3 vertebral body and erosion of the Th4 vertebral endplate were observed.
After one month (Fig. 1C), the anterior half of the Th3 vertebral body was fragmented into separate bone pieces, and pathological changes extended to the posterior part of the body. Negative changes were also noted in the Th4 vertebral body, though these were less pronounced.An analysis of the provided medical documentation revealed that C-reactive protein (CRP) levels post-injury did not exceed 4.8 mg/L, and peripheral blood leukocytes remained at 7.6 × 10¹²/L, ruling out a septic nature for the observed changes [19–21].

Fig. 1. Patient N., 50 years old. CT scans of the thoracic spine: A – on the day of the injury; B – after 2 weeks; C – after 1 month (details in the text)
Clinical case No.2
Patient T., a 41-year-old military serviceman, sustained injuries due to a fall on a flat surface caused by a blast wave, accompanied by a brief loss of consciousness. During the initial examination at the hospital, lower paraplegia and anesthesia of all types of sensation below the Th12–L1 level were detected. On the day of hospitalisation, a brain CT scan was performed, resulting in resection craniotomy and removal of an acute subdural hematoma in the left parietal region.
On the second day post-injury, CT scans of the cervical, thoracic, and lumbar spine were conducted. Diagnosed injuries included a compression fracture of the L1 vertebral body, fractures of the superior facet joints of L1, and the inferior facet joints of Th12, with the fracture line extending to the spinous process of Th12. Significant compression of spinal canal structures was identified due to anterolisthesis of the Th12 vertebral body, with an 11 mm forward displacement, corresponding to type C injuries (Fig. 2A).
On the seventh day of hospitalization, decompressive-stabilization surgery was planned but was not performed due to acute cardiac dysfunction during anesthetic management. The patient remained in the intensive care and resuscitation unit and later in the trauma department. After 1.5 months post-injury, the patient demonstrated recovery of proprioceptive elements in the lower limbs.
At the request of the patient's relatives, he was transferred to the Romodanov Institute of Neurosurgery, NAMS of Ukraine, for surgical intervention. During preparation for the transfer, a follow-up CT scan of the thoracolumbar spine was performed at the hospital, revealing significant lysis of the anterior two-thirds of the L1 vertebral body (Fig. 2B). Upon admission to the Romodanov Institute of Neurosurgery (2 months post-injury), a CT scan revealed almost complete lysis of the L1 vertebral body: the anterior sections were not visualized, and the posterior sections consisted of isolated bone fragments. Additionally, the process extended to the endplates of the adjacent vertebrae, Th11 and L2. At the time of admission, the leukocyte count was 8.8 × 10⁹/L, and the C-reactive protein (CRP) level was 3.1 mg/L. Bacteriological analysis of intraoperatively obtained samples (three specimens from the lysis zone of the L1 vertebral body) showed no microbial growth.
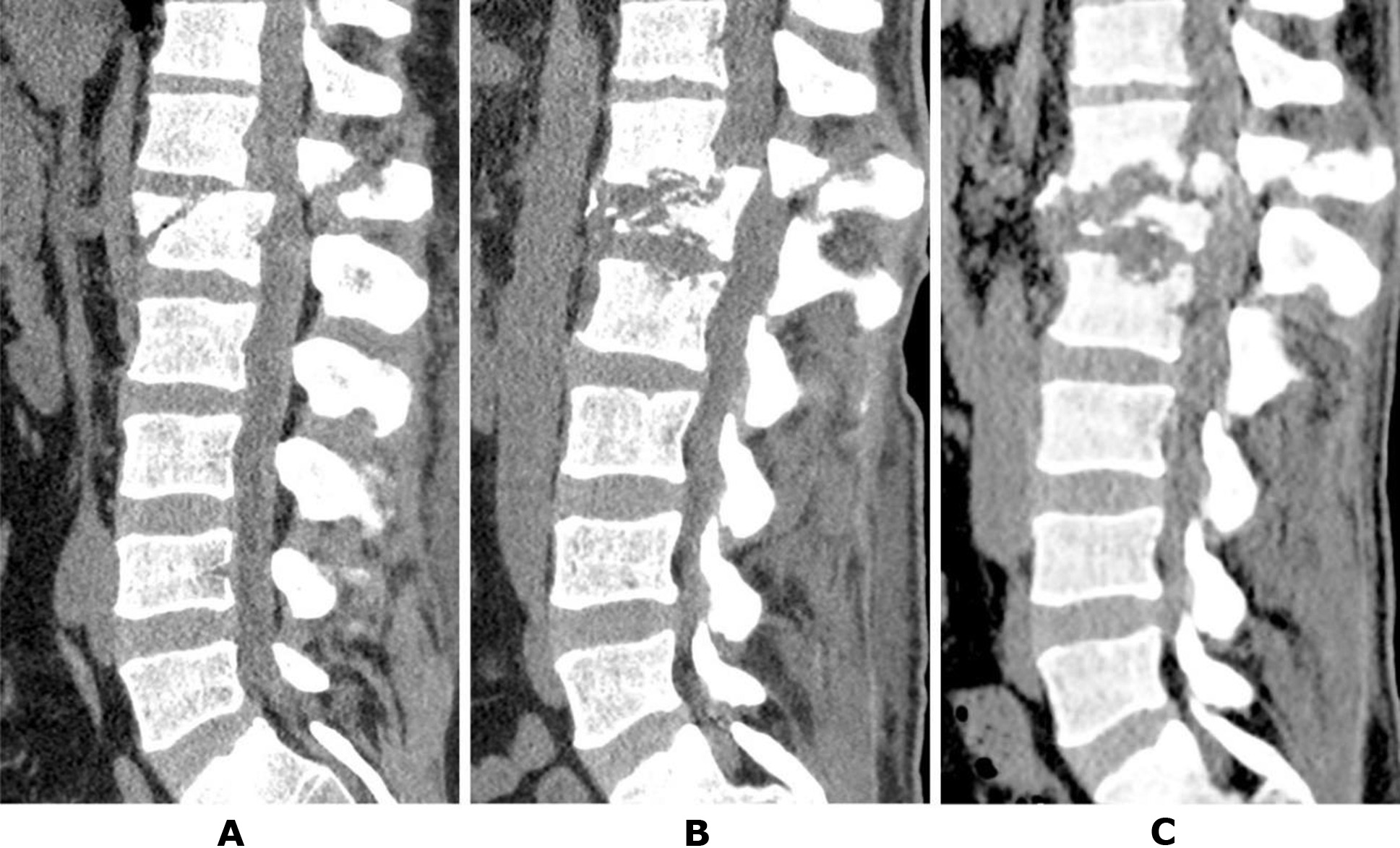
Fig. 2. Patient T., 41 years old. CT scans of the lumbar spine: A – the day after the injury; B – after 1.5 months; C – after 2 months post-injury (details in the text)
An analysis of the available clinical material did not support radiologically established instability as the primary predictor of aseptic spondylolysis. As an example, we present two cases where this pathology was not observed despite significant bone-destructive changes.
Clinical case No.3
Patient P., a 37-year-old woman, was injured in a road traffic accident as a passenger. Upon hospitalisation at a central district hospital, CT scans were performed, revealing a fracture of the Th7 vertebral body with significant destruction and fragment displacement into the spinal canal, as well as fractures of the arches and spinous processes of Th6 and Th7 vertebrae (Fig. 3A). Additionally, multiple rib fractures and hemopneumothorax were diagnosed. The neurological status was classified as ASIA A.
The patient remained in the intensive care unit for 3 weeks, followed by 2 weeks in the trauma department after stabilization of vital functions. She was then transferred to the Romodanov Institute of Neurosurgery, NAMS of Ukraine, for surgical treatment. At the time of transfer, the level of neurological deficit remained ASIA A. A CT scan performed during hospitalisation revealed progression of the deformity (Fig. 3B). The patient also presented with a sacral pressure ulcer measuring up to 10 cm (grade 2, proliferative stage). The leukocytosis count at the time of admission was 10.8 × 10⁹/L.
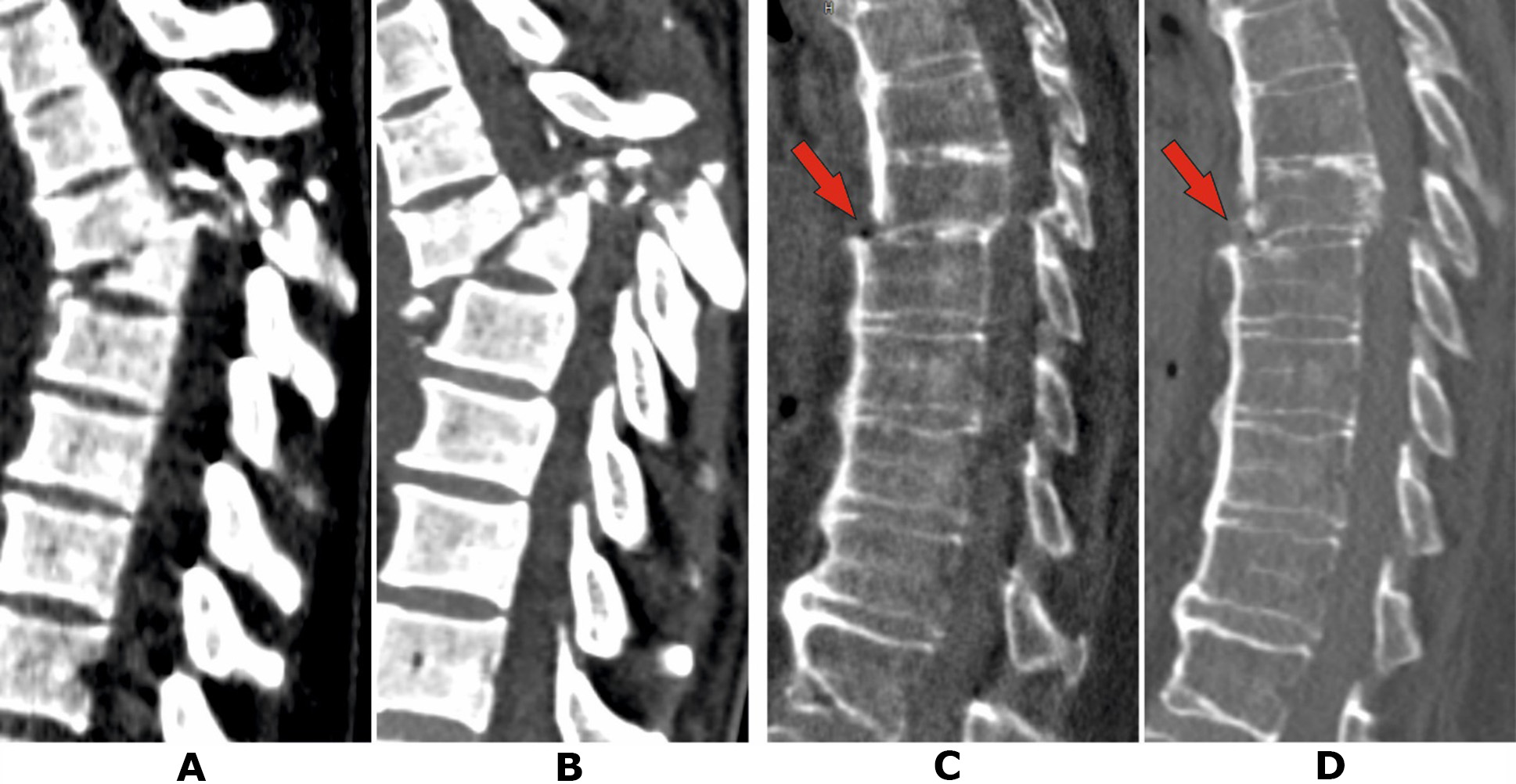
Fig. 3. Patient P., 37 years old. CT scans of the thoracic spine: A – on the day of the injury; B – after 5 weeks. Patient V., 58 years old: C – 2 days post-injury; D – after 2 months (details in the text)
Clinical Case No. 4
Patient V., a 58-year-old woman, was injured in a road traffic accident as a pedestrian. She was hospitalised in the local trauma department and diagnosed with fractures of both femurs and the right tibia. Neurological status at admission corresponded to ASIA E. Skeletal traction was applied. Due to complaints of back pain on the day following the injury, a CT scan of the thoracic spine was performed, revealing a distraction fracture in the Th7-Th8 segment against the background of localized thoracic ossification of the anterior longitudinal ligament (Fig. 3C). Given the minor displacement, preserved neurological functions, and the anticipated prolonged immobilisation period, stabilisation of the injury was not performed. The patient remained in the trauma department for 2 months and reported gradual reduction in back and lower limb pain. However, a scheduled neurological examination confirmed lower paraplegia and anesthesia at the Th8 level. She was transferred to the Romodanov Institute of Neurosurgery, NAMS of Ukraine, for surgical treatment. At the time of hospitalisation, CT scans showed no significant negative radiological changes compared to previous findings. Neurological status corresponded to ASIA A. Examination revealed a large sacral pressure ulcer exceeding 20 cm in diameter (grade 4 with necrotic areas). Peripheral blood leukocyte count at admission was 16.3 × 10⁹/L.
These clinical cases clearly demonstrate that the presence of chronic septic processes in patients is not a predictive factor for the development of post-traumatic ANVB. Furthermore, a retrospective analysis of medical records did not identify potential predictors for this pathological condition. Table 2 provides a comparative characterization of the group of patients with post-traumatic ANVB and the comparison group in which such changes were not observed despite unstable injuries.
Table 2. Comparison of key clinical parameters between the group of patients with aseptic post-traumatic vertebral body necrosis and the comparison group
|
Indicator |
Group with Spondylolysis |
Comparison Group |
P |
|
Sex: |
|
0,99∆ |
|
|
men |
66,67% |
61,54% |
|
|
women |
33,33% |
38,46% |
|
|
Age, years: |
|
|
0,5* |
|
median (95 % confidence interval) |
43,5 (25,25–58,08 |
40 (26,9–45,78) |
|
|
Injury Level: |
|
0,3∆ |
|
|
thoracic |
33,33% |
23,08% |
|
|
Thoracolumbar junction |
|
38,46% |
|
|
lumbar |
0% |
38,46% |
|
|
AOSpine injury type: |
|
0,99∆ |
|
|
B2 |
50% |
46,15% |
|
|
B3 |
0% |
7,69% |
|
|
C |
50% |
46,15% |
|
|
TLICS, score |
|
|
0,7* |
|
median (95 % confidence interval) |
8 (6,05‒8,95) |
8(6,38‒7,93) |
|
|
Neurological deficit (ASIA): |
|
0,4∆ |
|
|
A |
50,0% |
69,23% |
|
|
B |
33,33% |
7,69% |
|
|
E |
16,67% |
23,08% |
|
|
Presence of neurotrophic changes |
16,67% |
61,54 |
0,1∆ |
|
Peripheral blood leukocytes, 109/L |
|
|
0,01† |
|
mean (95% confidence interval) |
8,47 (5,79‒11,14) |
13,04 (10,3‒15,78) |
|
|
C-Reactive Protein (CRP), mg/L |
|
|
‒ |
|
mean (95% confidence interval) |
2,45 (0,96‒3,94) |
‒ |
|
Note: ∆ ‒ Fisher's exact test; * ‒ Fisher-Pitman permutation test for two samples; † ‒ Welch’s t-test.
It is noteworthy that stabilization surgery in all patients with post-traumatic necrosis associated with unstable spinal injuries completely prevented further progression of destructive processes. None of the presented clinical cases involved the use of osteotropic antibacterial therapy, underscoring the aseptic nature of the condition. For example, consider the follow-up data of patient T. (Case No. 2). Amid total lysis of the L1 vertebral body, rapid destruction of the endplates of the adjacent vertebrae was observed (see Figures 2B, 2C). However, following decompressive-stabilization surgery—including removal of L1 vertebral body fragments, placement of a body-replacement implant via a posterior approach, and subsequent transpedicular stabilization of the Th11-Th12-L2-L3 vertebrae—the process was fully arrested (Fig. 4A). Follow-up studies conducted 2 and 6 months after surgery demonstrated stable fixation and no signs of progressive osteolysis (Figures 4B, 4C).
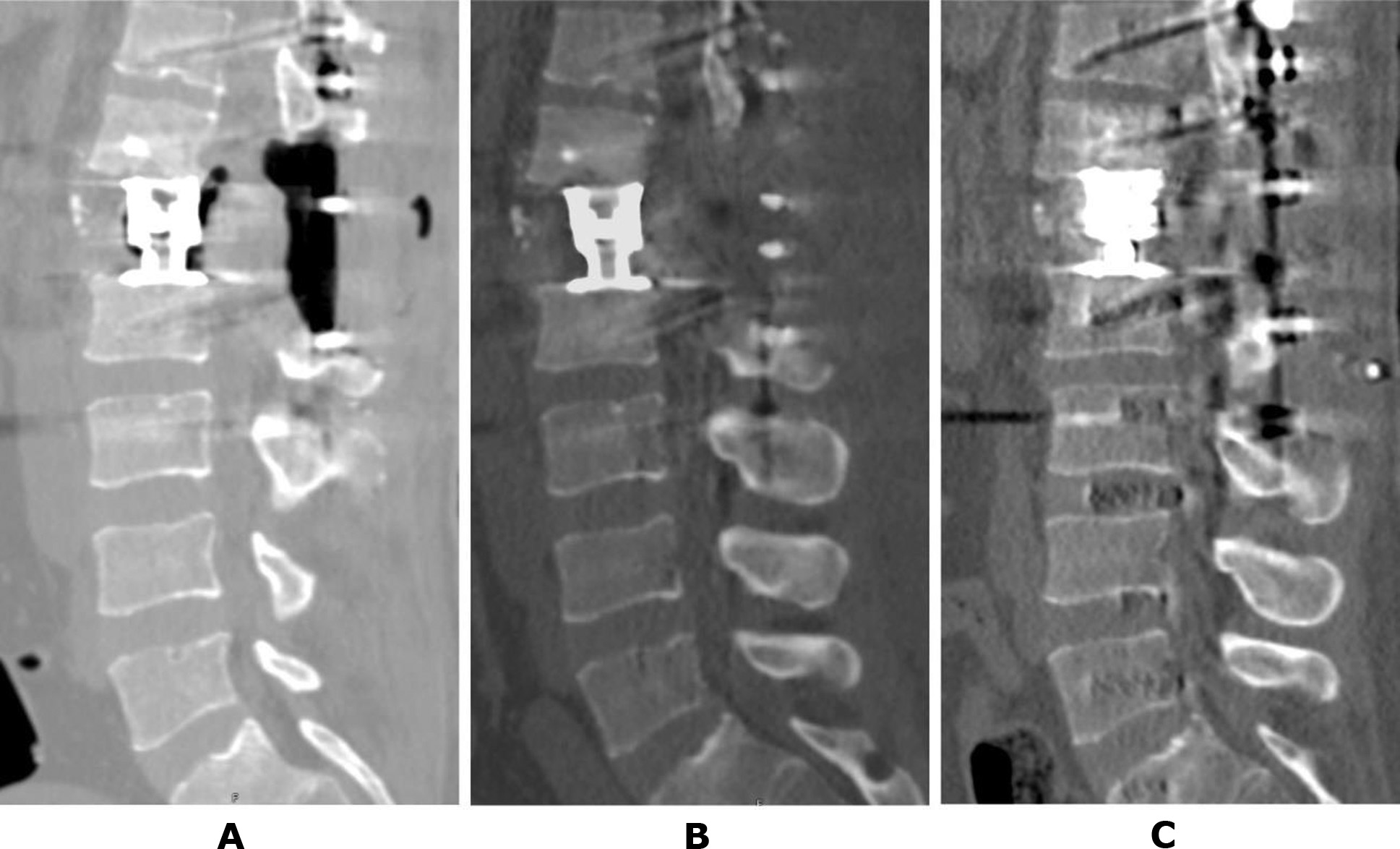
Fig. 4. Patient T., 41 years old. CT scan of the lumbar spine: A – 3 days after surgery; B – 2 months later; C – 6 months later (details provided in the text)
Discussion
Aseptic necrosis of vertebral bodies remains a subject of active study. The literature contains conflicting data regarding its pathogenesis and actual prevalence. As noted above, in most cases, references to aseptic necrosis of the vertebral bodies (ANVB) pertain to Kummell’s disease. However, the diagnostic criteria for this rare pathological condition have been significantly expanded, often unjustifiably, leading to a blurred clinical picture and complicating the understanding of its course and the development of optimal treatment methods [22]. According to some authors, the prevalence of Kummell’s disease is approximately 37% among all osteoporotic fractures in the elderly population, which suggests overdiagnosis [23].
Traditionally, Kummell’s disease is interpreted as a post-traumatic vertebral fracture that is asymptomatic and radiologically undetectable at initial stages but eventually leads to vertebral body collapse [24].
In 1951, H. Steel provided a detailed description of the stages of Kummell’s disease progression [25]. First stage: Initial trauma, which can vary in severity and mechanism, while the radiologically examined vertebra remains intact. Second stage: Post-traumatic phase, where patients may experience mild back pain without significant functional limitations. Third (latent) stage: May last from several weeks to months, often asymptomatic. Fourth stage (recurrence phase): Persistent and progressive back pain localized at the site of the forming compression fracture. Terminal stage: Gradual development of kyphotic deformity with potential spinal cord compression.
The presented data highlight the fundamental differences between the pathology discussed in this publication and Kummell’s disease. Given the similarity of pathological processes, specifically ANVB, and the lack of literature describing the pathological condition we observed, we explore the currently known potential mechanisms of aseptic spondylonecrosis pathogenesis.
There are numerous hypotheses explaining the development of aseptic necrosis of vertebral bodies (ANVB): avascular osteonecrosis [26–28], impaired consolidation due to atrophy [29], microfractures [24], tissue nourishment disorders [25], pseudoarthrosis [26], and stress fractures [30]. Identified risk factors include prolonged glucocorticoid therapy, venous stasis, diabetes, alcoholism, pancreatitis, radiation therapy, oncopathology, and chronic infections [31, 32]. Despite extensive studies of post-traumatic ANVB in general, and Kummell’s disease in particular, the cascade of pathological processes leading to vertebral body lysis remains unknown. Ischemia of the vertebral body is considered the most probable cause of ANVB development. Some authors suggest that avascular osteonecrosis of the vertebral body and Kummell’s disease are synonymous. According to current theories, ischemia may result from blood vessel rupture due to trauma, intravascular occlusion, or extravascular compression caused by increased interstitial pressure [33].
The nature of the initial traumatic injury to vertebral body blood vessels is closely related to the specifics of its blood supply. A review of the literature reveals limited research on blood flow in bony structures, as the most detailed publications on this topic date back to the 1960s–1980s. More recent studies predominantly focus on spinal cord blood supply, which understandably holds greater practical significance. Moreover, the lack of a clear nomenclature for small-caliber arteries further complicates comprehension [34]. Below is a fundamental description of the blood supply scheme necessary for understanding the theory of avascular spondylonecrosis.
It is known that the primary source of blood supply to the vertebral bodies of the mid-to-lower thoracic and lumbar regions is the segmental arteries, which include posterior intercostal arteries (aa. intercostales posteriores) and lumbar arteries (aa. lumbales), originating from the thoracic and abdominal sections of the aorta, respectively. In the thoracic region, the right segmental arteries are longer than the left due to the aorta's position along the anterolateral left surface of the spine at the arch, transitioning to a nearly central location in the abdominal section. As the segmental arteries curve around the vertebral body, they give rise to small branches—anterior and lateral vertebral branches—that perforate the cortical layer and contribute to forming the vertebral body vascular plexus. There are no reports suggesting direct blood supply to vertebral bodies from the aorta. On the lateral surface of the vertebral body, anastomoses link homonymous vessels located cranially and caudally, which also give rise to lateral vertebral branches. At both thoracic and lumbar levels, the segmental arteries give off a large dorsal branch (r. dorsalis = a. dorsospinalis) critical for the vascularization of bony and neural structures. The dorsal branch divides into r. retrovertebralis, r. spinalis, and r. muscularis. The retrovertebral branch, passing through the intervertebral foramen, encircles the vertebral body on its posterior surface, often forming anastomoses with the contralateral branch. Small vessels branching from it penetrate the cortical layer and participate in forming the vascular plexus of the vertebral body. Some authors consider the retrovertebral branch the primary source of vertebral body blood supply [35]. R. spinalis also courses dorsomedially, dividing into anterior and posterior radiculomedullary arteries (aa. radiculomedullaris ant. et post.), which supply the spinal canal's neural structures. R. muscularis, extending dorsally, supplies structures of the posterior supporting complex and the deep muscles of the back. Some authors distinguish medial, intermediate, and lateral branches of the r. muscularis [36].
Within the vertebral body, the vascular plexus—centered at the anatomical core of the vertebral body—is formed by arteries penetrating the cortical layer from the sources described earlier. Radiating outward in all directions, the vessels of the plexus anastomose with branches of penetrating arteries, creating both centrifugal and centripetal blood flow. The densest arterial network is visualized in the posterolateral regions of the vertebral body and its center [35, 37]. These features result in the formation of a watershed zone—a region of reduced blood supply located in the anterior third of the vertebral body. On sagittal sections, this watershed zone has a wedge shape directed from the center to the anterior surface of the vertebral body and is characterized by exclusively centrifugal blood flow, as penetrating arteries are virtually absent on the anterior surface [38]. Expectedly, this phenomenon becomes more pronounced the closer the aorta is to the central line—at the level of the arch, the thoracolumbar transition, and the lumbar region down to the bifurcation into the common iliac arteries.
The presented data demonstrate that, overall, the blood supply to the vertebral body has significant compensatory potential due to numerous anastomoses. Clinical observations indicate that even substantial damage and fragmentation of vertebral bodies rarely result in pronounced ischemia. However, the specifics of blood flow and vascularization in traumatically injured vertebrae have not been thoroughly studied. Consequently, blood flow disturbances resulting from traumatic injury or compression of adjacent vessels, either in isolation or combined with other factors, are considered potential causes of ANVB.
Blood flow disruption within the vertebral body may also occur due to intravascular occlusion. Cases of non-traumatic ANVB have been described in the literature in association with vaso-occlusive sickle cell crisis or decompression sickness [38,39]. It has been noted that pancreatitis is also a risk factor for ANVB development, as elevated levels of lipolytic enzymes in the blood lead to the breakdown of intramedullary fat structures and vascular obstruction by fat droplets. Additionally, instances of pancreatic enzyme release into the abdominal cavity following cyst rupture, leading to subsequent ANVB development, have been documented [40–42]. Arterial anomalies, dyslipidemia, leukemia, and lymphoma have also been linked to the development of spondylonecrosis [43, 44]. Regarding post-traumatic osteonecrosis, intravascular occlusion is likely a heightened risk factor for the group of patients under consideration [33].
Some authors consider extravascular obliteration caused by increased interstitial pressure as one of the links in the pathogenesis of ANVB [45]. Chronic glucocorticoid therapy and alcohol consumption, which lead to fat embolism, lipid deposition, and adipocyte hypertrophy, are also significant risk factors as noted in the literature [46–48]. Regarding the pathology we analyzed, it can be hypothesized that extravascular obliteration may contribute to the progression of spondylonecrosis. Specifically, primary traumatic damage to the vertebral body and the resulting disruption of blood flow due to vascular injury in the spongy bone leads to the formation of an ischemic zone. The situation is exacerbated by instability at the injury site, which causes increased fragment mobility and hinders neoangiogenesis. Bone tissue lysis is accompanied by the release of a significant amount of low-molecular-weight compounds into the interstitial space, negatively affecting the patency of intact vessels and leading to the gradual spread of pathological changes to unaffected areas of the body. Overall, the avascular theory of post-traumatic ANVB development aligns well with the clinical picture. In all documented cases, the lysis process began in the anterior parts of the vertebral body and gradually extended to the central and posterior regions. However, regarding Kummell's disease, the avascular mechanism is questioned by several researchers. The pathological cascade described struggles to explain the development of avascular osteonecrosis in patients 2–3 months after trauma, with no clinical manifestations or radiological changes at the onset [15, 49].
When considering the phenomenon of ANVB, it is important to mention the "vacuum effect," which has been increasingly recognized as a practically pathognomonic symptom of Kummell's disease in recent decades. Gas formation is attributed to tissue breakdown and the release of gas as a result of necrotic processes. The decomposition of proteins, lipids, and other components of necrotic tissues releases gases such as nitrogen, oxygen, and carbon dioxide, which then accumulate in the vertebra or disc region. T. Armingeat et al. analyzed the composition of the gas responsible for the vacuum effect and found that 90–92% of it is nitrogen [50]. On the other hand, some authors note that tissue necrosis leads to changes in intravertebral pressure, causing gas diffusion from the blood into bone structures [46]. However, this excludes the possible influence of increased interstitial pressure on the development of ANVB.
An analysis of the literature on the vacuum effect revealed that its occurrence is inversely proportional to the bone density of the affected vertebra [32]. Since in the last decade there has been a tendency to consider osteoporotic fractures as Kummell's disease, the presence of gas in the vertebral body or intervertebral disc has been given excessive diagnostic significance. It is known that the presence of gas in a compression fracture can be a radiological finding and does not necessarily indicate progressive bone tissue destruction [51, 52]. The vacuum phenomenon is often observed in degenerative spinal diseases [53–56]. In our group of patients with documented ANVB phenomena, the vacuum phenomenon was not observed in any case.
As noted above, the role of injury instability in the development of ANVB has not been adequately studied. However, concerning traumatic injuries in other parts of the skeleton, this issue has been much better addressed. Several publications demonstrate a clear relationship between instability, the timing of its resolution, and the frequency of osteonecrosis [57–59], which to some extent supports the validity of our assumptions. The data we have presented on the possible pathophysiological mechanisms underlying this phenomenon allow differentiation from Kummell's disease and highlight areas for further research and prevention of this significant complication.
A notable limitation of this study is its retrospective nature and the small number of patients, which prevented us from identifying precise predictors of ANVB development in cases of unstable vertebral injury. Furthermore, the interpretation and subsequent formation of a comparison group are complicated by the definition of instability. While often effective in the context of overall functional activity, this definition does not fully characterize the biomechanical state of the damaged spinal motion segment (SMS). This can be illustrated by the example of the cervical spine, where a complete locked dislocation is, by definition, unstable but simultaneously rigidly fixed in terms of the range of motion in the damaged SMS. A similar situation is sometimes observed in the thoracolumbar spine and is well known to practicing surgeons: despite a significant degree of injury, fragmented structures of the posterior supporting complex, due to fragment interlocking, may hinder further displacement and even completely immobilize the segment. This factor should also be considered in future research.
Conclusions
This article is one of the first dedicated to describing the practically unexplored phenomenon of post-traumatic ANVB in unstable injuries of the thoracolumbar spine. The presented data, along with the consideration of possible pathogenetic mechanisms, clearly highlight the importance of early stabilization of unstable injuries not only from a neurological perspective but also from an orthopedic standpoint. This information may be valuable for practicing neurosurgeons or orthopedic trauma specialists, especially given the high incidence of traumatic injuries in the population.
Disclosure
Conflict of Interest
The authors declare no conflict of interest.
Informed Consent
Informed consent was obtained from all patients.
Funding
The study received no sponsorship or financial support.
References
1. White AA, Southwick WO, Panjabi MM. Clinical Instability in the Lower Cervical Spine A Review of Past and Current Concepts. Spine. 1976;1.
2. White AA, Panjabi MM. Clinical Biomechanics of the Spine: Lippincott; 1990.
3. Panjabi MM, Brand RA Jr, White AA 3rd. Three-dimensional flexibility and stiffness properties of the human thoracic spine. J Biomech. 1976;9(4):185-92. https://doi.org/10.1016/0021-9290(76)90003-8
4. Pope MH, Panjabi M. Biomechanical definitions of spinal instability. Spine (Phila Pa 1976). 1985 Apr;10(3):255-6. https://doi.org/10.1097/00007632-198504000-00013
5. Liebsch C, Wilke HJ. Which traumatic spinal injury creates which degree of instability? A systematic quantitative review. Spine J. 2022 Jan;22(1):136-156. https://doi.org/10.1016/j.spinee.2021.06.004
6. Kim CW, Perry A, Garfin SR. Spinal instability: the orthopedic approach. Semin Musculoskelet Radiol. 2005 Mar;9(1):77-87. https://doi.org/10.1055/s-2005-867098
7. Izzo R, Guarnieri G, Guglielmi G, Muto M. Biomechanics of the spine. Part II: spinal instability. Eur J Radiol. 2013 Jan;82(1):127-38. https://doi.org/10.1016/j.ejrad.2012.07.023
8. Izzo R, Guarnieri G, Muto M. Stability and Instability of the Spine. In: Manfrè L, editor. Spinal Instability. Cham: Springer International Publishing; 2015. p. 1-26.
9. Maschmann C, Jeppesen E, Rubin MA, Barfod C. New clinical guidelines on the spinal stabilisation of adult trauma patients - consensus and evidence based. Scand J Trauma Resusc Emerg Med. 2019 Aug 19;27(1):77. https://doi.org/10.1186/s13049-019-0655-x
10. Abbasi Fard S, Skoch J, Avila MJ, Patel AS, Sattarov KV, Walter CM, Baaj AA. Instability in Thoracolumbar Trauma: Is a New Definition Warranted? Clin Spine Surg. 2017 Oct;30(8):E1046-E1049. https://doi.org/10.1097/BSD.0000000000000314
11. Blair JA, Patzkowski JC, Schoenfeld AJ, Cross Rivera JD, Grenier ES, Lehman RA Jr, Hsu JR; Skeletal Trauma Research Consortium (STReC). Spinal column injuries among Americans in the global war on terrorism. J Bone Joint Surg Am. 2012 Sep 19;94(18):e135(1-9). https://doi.org/10.2106/JBJS.K.00502
12. Owens BD, Kragh JF Jr, Wenke JC, Macaitis J, Wade CE, Holcomb JB. Combat wounds in operation Iraqi Freedom and operation Enduring Freedom. J Trauma. 2008 Feb;64(2):295-9. https://doi.org/10.1097/TA.0b013e318163b875
13. Blair JA, Patzkowski JC, Schoenfeld AJ, Cross Rivera JD, Grenier ES, Lehman RA, Hsu JR; Skeletal Trauma Research Consortium (STReC). Are spine injuries sustained in battle truly different? Spine J. 2012 Sep;12(9):824-9. https://doi.org/10.1016/j.spinee.2011.09.012
14. Bernstock JD, Caples CM, Wagner SC, Kang DG, Lehman RA Jr. Characteristics of combat-related spine injuries: a review of recent literature. Mil Med. 2015 May;180(5):503-12. https://doi.org/10.7205/MILMED-D-14-00215
15. Li H, Liang CZ, Chen QX. Kümmell’s disease, an uncommon and complicated spinal disorder: a review. J Int Med Res. 2012;40(2):406-14. https://doi.org/10.1177/147323001204000202
16. Maynard FM Jr, Bracken MB, Creasey G, Ditunno JF Jr, Donovan WH, Ducker TB, Garber SL, Marino RJ, Stover SL, Tator CH, Waters RL, Wilberger JE, Young W. International Standards for Neurological and Functional Classification of Spinal Cord Injury. American Spinal Injury Association. Spinal Cord. 1997 May;35(5):266-74. https://doi.org/10.1038/sj.sc.3100432
17. Vaccaro AR, Oner C, Kepler CK, Dvorak M, Schnake K, Bellabarba C, Reinhold M, Aarabi B, Kandziora F, Chapman J, Shanmuganathan R, Fehlings M, Vialle L; AOSpine Spinal Cord Injury & Trauma Knowledge Forum. AOSpine thoracolumbar spine injury classification system: fracture description, neurological status, and key modifiers. Spine (Phila Pa 1976). 2013 Nov 1;38(23):2028-37. https://doi.org/10.1097/BRS.0b013e3182a8a381
18. Vaccaro AR, Lehman RA Jr, Hurlbert RJ, Anderson PA, Harris M, Hedlund R, Harrop J, Dvorak M, Wood K, Fehlings MG, Fisher C, Zeiller SC, Anderson DG, Bono CM, Stock GH, Brown AK, Kuklo T, Oner FC. A new classification of thoracolumbar injuries: the importance of injury morphology, the integrity of the posterior ligamentous complex, and neurologic status. Spine (Phila Pa 1976). 2005 Oct 15;30(20):2325-33. https://doi.org/10.1097/01.brs.0000182986.43345.cb
19. Herren C, Jung N, Pishnamaz M, Breuninger M, Siewe J, Sobottke R. Spondylodiscitis: Diagnosis and Treatment Options. Dtsch Arztebl Int. 2017 Dec 25;114(51-52):875-882. https://doi.org/10.3238/arztebl.2017.0875
20. Leal FS, de Tella OI Jr, Bonatelli Ade P, Herculano MA, Aguiar PH. Espondilodiscites sépticas: diagnóstico e tratamento [Septic spondylodiscitis: diagnosis and treatment]. Arq Neuropsiquiatr. 2003 Sep;61(3B):829-35. Portuguese. https://doi.org/10.1590/s0004-282x2003000500023
21. Salaffi F, Ceccarelli L, Carotti M, Di Carlo M, Polonara G, Facchini G, Golfieri R, Giovagnoni A. Differentiation between infectious spondylodiscitis versus inflammatory or degenerative spinal changes: How can magnetic resonance imaging help the clinician? Radiol Med. 2021 Jun;126(6):843-859. https://doi.org/10.1007/s11547-021-01347-7
22. Alcázar Parra A, Campos García J. Necrosis avascular vertebra.: Una patología infradiagnosticada. Seram. 2018.
23. Lee SM, Oh HS, Lee SH, Lee HC, Hwang BW. Cement Augmented Anterior Reconstruction and Decompression without Posterior Instrumentation: A Less Invasive Surgical Option for Osteoporotic Thoracolumbar Fracture with Cord Compression. Korean J Neurotrauma. 2020 Oct 21;16(2):190-199. https://doi.org/10.13004/kjnt.2020.16.e37
24. Benedek TG, Nicholas JJ. Delayed traumatic vertebral body compression fracture; part II: pathologic features. Semin Arthritis Rheum. 1981 May;10(4):271-7. https://doi.org/10.1016/0049-0172(81)90004-4
25. STEEL HH. Kümmell’s disease. Am J Surg. 1951 Feb;81(2):161-7. https://doi.org/10.1016/0002-9610(51)90206-1
26. Jang JS, Kim DY, Lee SH. Efficacy of percutaneous vertebroplasty in the treatment of intravertebral pseudarthrosis associated with noninfected avascular necrosis of the vertebral body. Spine (Phila Pa 1976). 2003 Jul 15;28(14):1588-92. https://doi.org/10.1097/01.brs.0000076824.61074.06
27. Huang SL, Shi W, He XJ. Avascular necrosis of a vertebral body. Chin J Traumatol. 2009 Apr;12(2):125-8. https://doi.org/10.3760/CMA.J.ISSN.1008-1275.2009.02.013
28. Chou LH, Knight RQ. Idiopathic avascular necrosis of a vertebral body. Case report and literature review. Spine (Phila Pa 1976). 1997 Aug 15;22(16):1928-32. https://doi.org/10.1097/00007632-199708150-00024
29. Freedman BA, Heller JG. Kummel disease: a not-so-rare complication of osteoporotic vertebral compression fractures. J Am Board Fam Med. 2009 Jan-Feb;22(1):75-8. https://doi.org/10.3122/jabfm.2009.01.080100
30. Brower AC, Downey EF Jr. Kümmell disease: report of a case with serial radiographs. Radiology. 1981 Nov;141(2):363-4. https://doi.org/10.1148/radiology.141.2.7291557
31. Lee SH, Kim ES, Eoh W. Cement augmented anterior reconstruction with short posterior instrumentation: a less invasive surgical option for Kummell’s disease with cord compression. J Clin Neurosci. 2011 Apr;18(4):509-14. https://doi.org/10.1016/j.jocn.2010.07.139
32. Wu AM, Chi YL, Ni WF. Vertebral compression fracture with intravertebral vacuum cleft sign: pathogenesis, image, and surgical intervention. Asian Spine J. 2013 Jun;7(2):148-55. https://doi.org/10.4184/asj.2013.7.2.148
33. Formica M, Zanirato A, Cavagnaro L, Basso M, Divano S, Formica C, Felli L. What is the Current Evidence on Vertebral Body Osteonecrosis?: A Systematic Review of the Literature. Asian Spine J. 2018 Jun;12(3):586-599. https://doi.org/10.4184/asj.2018.12.3.586
34. Campos Moraes Amato A, Groppo Stolf NA. Anatomy of spinal blood supply. Jornal Vascular Brasileiro. 2015;14(3):248-252. https://doi.org/10.1590/1677-5449.0004
35. Crock HV, Yoshizawa H. The blood supply of the lumbar vertebral column. Clin Orthop Relat Res. 1976 Mar-Apr;(115):6-21. https://doi.org/10.1097/00003086-197603000-00003
36. Chiras J, Morvan G, Merland JJ, Bories J. Blood supply to the thoracic (dorsal) and lumbar spine. Anatomia Clinica. 1982;4(1):23-31. https://doi.org/10.1007/BF01811186.
37. Prakash, Prabhu LV, Saralaya VV, Pai MM, Ranade AV, Singh G, Madhyastha S. Vertebral body integrity: a review of various anatomical factors involved in the lumbar region. Osteoporos Int. 2007 Jul;18(7):891-903. https://doi.org/10.1007/s00198-007-0373-5
38. Maheshwari PR, Nagar AM, Prasad SS, Shah JR, Patkar DP. Avascular necrosis of spine: a rare appearance. Spine (Phila Pa 1976). 2004 Mar 15;29(6):E119-22. https://doi.org/10.1097/01.brs.0000115139.07685.ee
39. Hutter CD. Dysbaric osteonecrosis: a reassessment and hypothesis. Med Hypotheses. 2000 Apr;54(4):585-90. https://doi.org/10.1054/mehy.1999.0901
40. Allen BL, Jr., Jinkins WJ, 3rd. Vertebral osteonecrosis associated with pancreatitis in a child. A case report. J Bone Joint Surg Am. 1978;60(7):985-987. https://doi.org/10.2106/00004623-197860070-00020
41. Baba T, Shitoto K, Yoshioka C, Kaneko H. Pathological fracture due to vertebral osteonecrosis associated with pancreatitis. Arch Orthop Trauma Surg. 2011 Jan;131(1):11-4. https://doi.org/10.1007/s00402-010-1087-2
42. Morita O, Ogose A, Hotta T, Kawashima H, Higuchi T, Suzuki K, Endo N. Pathological fractures due to intraosseous fat necrosis associated with pancreatitis. Rheumatology (Oxford). 2003 Feb;42(2):394-6. https://doi.org/10.1093/rheumatology/keg086
43. Van Eenenaam DP, el-Khoury GY. Delayed post-traumatic vertebral collapse (Kummell’s disease): case report with serial radiographs, computed tomographic scans, and bone scans. Spine (Phila Pa 1976). 1993 Jul;18(9):1236-41. https://doi.org/10.1097/00007632-199307000-00019
44. Wang F, Wang D, Tan B, Dong J, Feng R, Yuan Z, Wang N. Comparative Study of Modified Posterior Operation to Treat Kümmell’s Disease. Medicine (Baltimore). 2015 Sep;94(39):e1595. https://doi.org/10.1097/MD.0000000000001595
45. Saran S, Rauniyar A, Madhuri BV, Kundu P. Multilevel Vertebral Body Osteonecrosis in an Adult Patient: A Rare Case with Review of Literature. J Assoc Physicians India. 2024 Oct;72(10):96-98. https://doi.org/10.59556/japi.72.0640
46. Osterhouse MD, Kettner NW. Delayed posttraumatic vertebral collapse with intravertebral vacuum cleft. J Manipulative Physiol Ther. 2002 May;25(4):270-5. https://doi.org/10.1067/mmt.2002.123164
47. Shah KN, Racine J, Jones LC, Aaron RK. Pathophysiology and risk factors for osteonecrosis. Curr Rev Musculoskelet Med. 2015 Sep;8(3):201-9. https://doi.org/10.1007/s12178-015-9277-8
48. Lafforgue P. Pathophysiology and natural history of avascular necrosis of bone. Joint Bone Spine. 2006 Oct;73(5):500-7. https://doi.org/10.1016/j.jbspin.2006.01.025
49. Javier RM, Moser T, Dietemann JL, Sparsa L, Natarajan-Ame S, Chenard MP, Kuntz JL. Multiple vertebral osteonecrosis. Joint Bone Spine. 2008 May;75(3):341-4. https://doi.org/10.1016/j.jbspin.2007.05.010
50. Armingeat T, Pham T, Legre V, Lafforgue P. Coexistence of intravertebral vacuum and intradiscal vacuum. Joint Bone Spine. 2006 Jul;73(4):428-32. https://doi.org/10.1016/j.jbspin.2005.10.011
51. Schömig F, Palmowski Y, Nikiforov I, Hartwig T, Pumberger M, Schwabe P, Jacobs C. Burst fractures lead to a fracture-associated intervertebral vacuum phenomenon: a case series of 305 traumatic fractures of the thoracolumbar spine. Eur Spine J. 2021 Oct;30(10):3068-3073. https://doi.org/10.1007/s00586-020-06590-6
52. Sasagawa T, Hayashi H, Takagi Y. Factors Associated with Intradiscal Vacuum Phenomenon after Traumatic Thoracolumbar Fracture. Asian J Neurosurg. 2023 Sep 27;18(3):621-625. https://doi.org/10.1055/s-0043-1775551
53. Liu Y, Chen L, Gu Y, Yang H, Tang T. [Research progress of vacuum phenomenon in spine]. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2011 Jan;25(1):96-9. Chinese.
54. Ekşi MŞ, Özcan-Ekşi EE, Akkaş A, Orhun Ö, Arslan HN, Zarbizada M, Küçüksüleymanoğlu D, Pamir MN, Benzel EC. Intradiscal vacuum phenomenon and spinal degeneration: a cross-sectional analysis of 219 subjects. Curr Med Res Opin. 2022 Feb;38(2):255-263. https://doi.org/10.1080/03007995.2021.1994379
55. Buttiens A, Simko M, Van Goethem J. Vacuum Phenomenon in the Lumbar Spine: Pilot Study for Accuracy of Magnetic Resonance Imaging. J Belg Soc Radiol. 2023 Nov 2;107(1):83. https://doi.org/10.5334/jbsr.3118
56. Cawley DT, Simpkin A, Abrahim E, Doyle T, Elsheikh N, Fallon J, Habash M, Phua RJ, Langille J, Matini E, McNamee C, Mohamed F, Gabhann CN, Noorani A, Oh J, O’Reilly P, O’Sullivan D, Devitt A. Intradiscal vacuum phenomenon matches lumbar spine degeneration patterns in an ageing population. Eur Spine J. 2024 May;33(5):2014-2021. https://doi.org/10.1007/s00586-024-08174-0
57. Léduc S, Clare MP, Laflamme GY, Walling AK. Posttraumatic avascular necrosis of the talus. Foot Ankle Clin. 2008 Dec;13(4):753-65. https://doi.org/10.1016/j.fcl.2008.09.004
58. Large TM, Adams MR, Loeffler BJ, Gardner MJ. Posttraumatic Avascular Necrosis After Proximal Femur, Proximal Humerus, Talar Neck, and Scaphoid Fractures. J Am Acad Orthop Surg. 2019 Nov 1;27(21):794-805. https://doi.org/10.5435/JAAOS-D-18-00225
59. Milenkovic S, Mitkovic M, Mitkovic M. Avascular necrosis of the femoral head after traumatic posterior hip dislocation with and without acetabular fracture. Eur J Trauma Emerg Surg. 2022 Feb;48(1):613-619. https://doi.org/10.1007/s00068-020-01495-x