Original article
Ukrainian Neurosurgical Journal. 2024;30(4):51-56
https://doi.org/10.25305/unj.312398
Extracerebral Tumor Department, Romodanov Neurosurgery Institute, Kyiv, Ukraine
Received: 29 September 2024
Accepted: 04 November 2024
Address for correspondence:
Anatoliy V. Spiridonov, Extracerebral Tumor Department, Romodanov Neurosurgery Institute, 32 Platona Mayborody st., Kyiv, 04050, Ukraine, e-mail: av.spiridonov0202@gmail.com
Objective: To investigate the impact of the degree of invasion of the superior sagittal sinus by meningiomas on the radicality of removal and to assess the risks of complications during surgical intervention for superior sagittal sinus meningiomas.
Materials and Methods: The study included 82 patients who underwent surgery at the Romodanov Neurosurgery Institute over the past 10 years (from 2013 to 2023). The cohort comprised 53 women and 29 men, with an average age of 43.4±1.7 years. Inclusion criteria are: a histologically confirmed diagnosis of meningioma and evidence of superior sagittal sinus invasion based on neuroimaging (MRI with intravenous contrast enhancement, MSCT angiography).
Results: A total of 84 surgical procedures were performed on 82 patients. Among these, 71 were primary cases (84.5%), and 13 were secondary cases (15.5%). In 7 out of 13 secondary surgeries, superior sagittal sinus invasion was first detected through neuroimaging and confirmed intraoperatively. Postoperative hemiparesis of varying degrees was observed in 41 patients (50%), with 10 cases showing an increase in neurological deficits due to surgical intervention. Motor deficits completely regressed within 3-6 months post-surgery in 28 out of 41 patients. Tumor recurrence was identified in 4 patients (4.9%) within 2.5-6 years after the primary surgery. Among these, 3 were morphologically confirmed as "anaplastic meningioma Grade III," and 1 as "atypical meningioma Grade II".
Conclusions: Meningiomas originating from the arachnoid membrane constitute a significant proportion of primary intracranial tumors, with varying degrees of venous sinus invasion. Surgical planning for meningiomas invading the superior sagittal sinus should consider the radiological classification of invasion degrees, which aids in determining the treatment strategy. MRI with intravenous contrast and MSCT angiography are crucial for identifying collateral blood flow and assessing the degree of venous sinus invasion before surgical intervention.
Keywords: meningioma; superior sagittal sinus; angiography; hemiparesis
Introduction
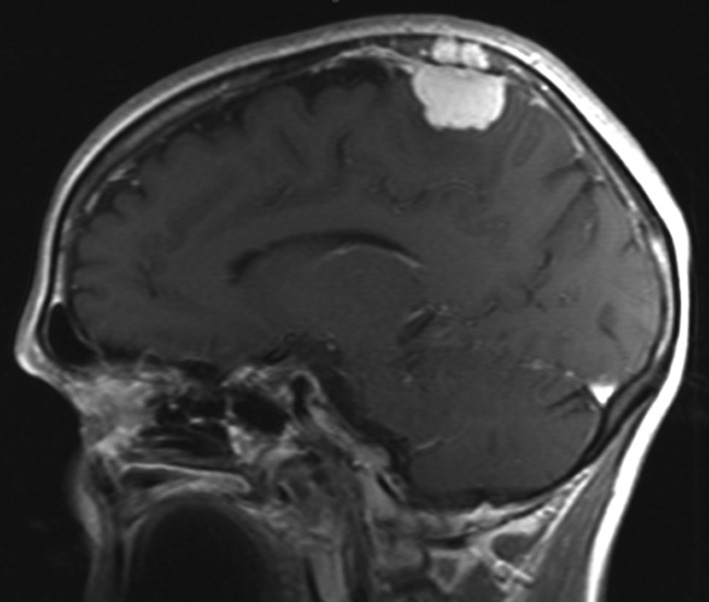
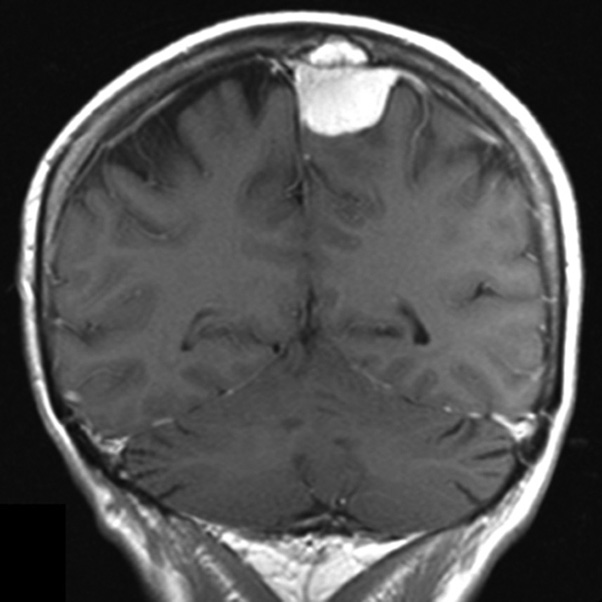
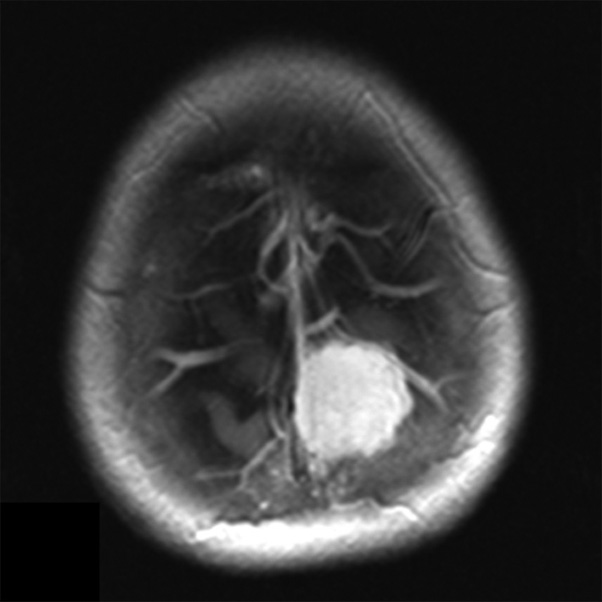
Meningiomas are benign tumors originating from the arachnoid membrane and account for 14–19% of all primary intracranial neoplasms [1]. Neurosurgical treatment of meningiomas that invade the sagittal sinus and cause partial or complete occlusion is a challenging task [2]. One aspect of the diagnostic problem for meningiomas in the sagittal sinus region is the preoperative determination of their relationship with the major brain arteries and the dura mater sinuses. Neuroimaging techniques, such as contrast-enhanced magnetic resonance imaging (MRI) (Fig. 1) and multislice computed tomography (MSCT) angiography (Fig. 2), enable the assessment of the tumor's relationship with the major arteries and venous sinuses.



Fig. 1. Brain MRI. Meningiomas of the mid-posterior third of the superior SSS in T1-weighted contrast-enhanced mode: A - sagittal projection, B - frontal projection, C - axial projection
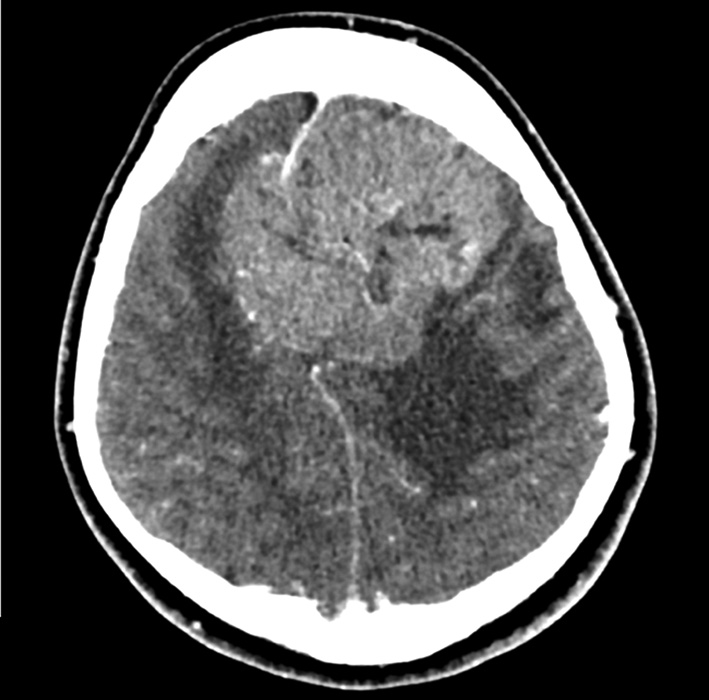
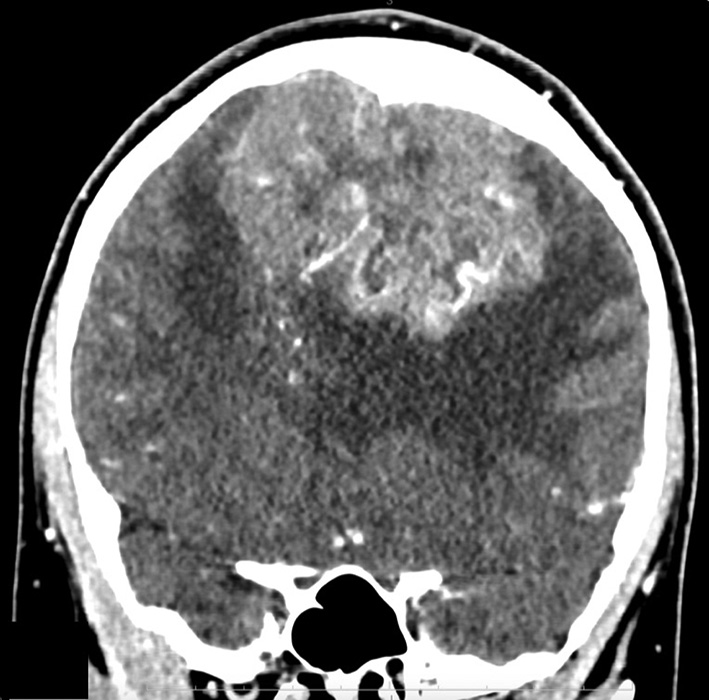
Fig. 2. MSCT - angiography. Giant meningioma of the anterior and middle third of the SSS with total occlusion of the superior sagittal sinus in the venous phase: A - axial projection; B - frontal projection
A retrospective comparison of angiographic data with intraoperative and pathological findings has identified a number of characteristic radiological features that allow the determination of the relationship between meningiomas in the sagittal sinus region and the major brain arteries and venous sinuses in the preoperative period. This issue is also complicated by the frequent involvement of draining veins in the tumor, whose injury can lead to the appearance or worsening of neurological deficits in patients. Aggressive removal of these tumors may result in sinus thrombosis, leading to venous infarction and worsening neurological symptoms depending on the location of the meningioma.
Materials and Methods
Study participants
The study included 82 patients operated on at the Romodanov Neurosurgery Institute during the last 10 years (2013–2023). There were 53 women, and 29 men. The mean age of the patients was (43.4±1.7) years.
The study was approved by the ethics and bioethics committee of the Romodanov Institute of Neurosurgery, National Academy of Medical Sciences of Ukraine (minutes No. 3 dated December 16, 2020). All participants provided informed and voluntary written consent for participation in the study and publication of data.
Inclusion Criteria
1) A histologically confirmed diagnosis of meningioma.
2) Evidence of invasion into the superior sagittal sinus (SSS) based on neuroimaging data (contrast-enhanced MRI, MSCT angiography).
3) Surgical intervention for a meningioma involving the superior sagittal sinus.
Group Characteristics
Type I invasion of meningioma of the superior sagittal sinus (SSS) by the classification of M.P. Sindou and J.E. Alvernia was detected in 50 (61.0%) patients, type ІІ - in 9 (11.0%), type ІІІ - in 5 (6.0%), type ІV - in 5 (6%), type V - in 6 (7.3%) and type VI - in 7 (8.7%).
Most meningiomas (71%) of the superior sagittal sinus site were located in the middle third of the sinus, corresponding to the segment from the coronal to the lambdoid suture, consistent with literature data (45.0–70.5%). Meningiomas in the anterior third of the sinus (from the crista galli to the coronal suture) accounted for 22% (18 patients), while those in the posterior third of the sinus accounted for 7% (6 patients).
In 68 cases (81%), a conservative approach was employed involving coagulation of the falx cerebri. In 16 cases (19%), an aggressive approach was taken, including resection of the affected portion of the falx cerebri, with resection of the affected SSS segment in 12 cases (75%).
Study Design
The study is retrospective.
Statistical Analysis
Data processing and analysis were performed using descriptive statistics, univariate and multivariate analysis, and survival assessment methods. The following software was used: Statistica v.10 (StatSoft® Inc., USA, license No. STA862D175437Q), SPSS 17.0 (IBM, USA), MedCalc (MedCalc Software Ltd, Ostend, Belgium; www.medcalc.org, trial versions 20.113 (2022) and 20.218 (2023)) The Shapiro-Wilk test was applied to verify the conformity of quantitative variables to a normal distribution. For normally distributed data, parametric statistics were used, including: mean (M), standard error of the mean (m), standard deviation (SD), comparisons were made using Student’s t-tests for independent (t) and dependent (T) samples. The statistical significance of differences in categorical data was assessed using Pearson’s χ² test.
Results
One of the critical factors to consider during surgical planning is the presence of collateral circulation developed during tumor growth [3]. In our practice, in addition to contrast-enhanced MRI, MR angiography or MSCT angiography was frequently employed for surgical planning. In 73% of patients, collateral blood flow was detected via magnetic resonance venography in cases of superior sagittal sinus occlusion.
Lesions of the SSS with complete lumen occlusion were identified in 6 cases via direct sinusography.
In 5 cases, a local filling defect of the SSS was recorded at the site of partial invasion of its lumen by the tumor. Indirect signs suggestive of potential tumor invasion into the SSS wall include the vascular shadow of the tumor in the sinus projection area [4]. Another diagnostic indicator is the outward deviation of the peripheral ascending branches of the callosomarginal artery. This sign indicates meningioma infiltration into the parasagittal angle, implying contact with the SSS wall. This is further supported by the displacement of the A4–A5 segments of the anterior cerebral artery across the midline.
Thus, careful examination of angiograms in patients with SSS meningiomas allows surgeons to preoperatively assess the degree of involvement of major brain vessels and dura mater sinuses in the tumor process.
Based on the obtained data, the following focal craniographic signs of intracranial meningiomas indicate the tumor's proximity to the bone: productive changes such as hyperostosis, bone thickening characterized by "swelling with irregular trabecular remodeling," and bone hypervascularization primarily due to the expansion and increased number of diploic channels. These features are diagnostically significant as they provide insights into both the localization and type of tumor. These signs are most commonly associated with meningiomas located on the convexity and, to a lesser extent, the basal surfaces of the cerebral hemispheres. It is important to note that hyperostosis (often diffuse) may sometimes be the only radiological sign of intraosseous or "en plaque" meningiomas [5].
Osteolytic changes such as thinning, protrusion, or erosion of the bone also suggest proximity to the tumor. However, these changes may also occur in other benign neoplasms. Bone resorption or osteoporosis, particularly in the convexity regions of the skull, can result not only from tumor effects but also from prolonged intracranial hypertension. Therefore, evaluating these signs requires considering clinical manifestations as well.
Radiological classification of venous sinus invasion:
Group 1 - partial sinus occlusion (<50%)
Group 2 - subtotal sinus occlusion (50–99%)
Group 3 - total venous sinus occlusion
To determine the degree of invasion using this classification, intravenous-enhanced MRI is used to assess meningioma spread, complemented by MSCT angiography (Table 1).
Table 1. Distribution of patients by degree of superior sagittal sinus invasion in this study
|
Angiographic type |
Degree of sinus invasion |
Number of patients |
|
Group 1 |
<50% |
49 (59,8%) |
|
Group 2 |
50‒99% |
11 (13,4%) |
|
Group 3 |
Total |
22 (26,8%) |
Patients were also classified according to the widely accepted classification by M.P. Sindou and J.E. Alvernia (Figs. 3 and 4).
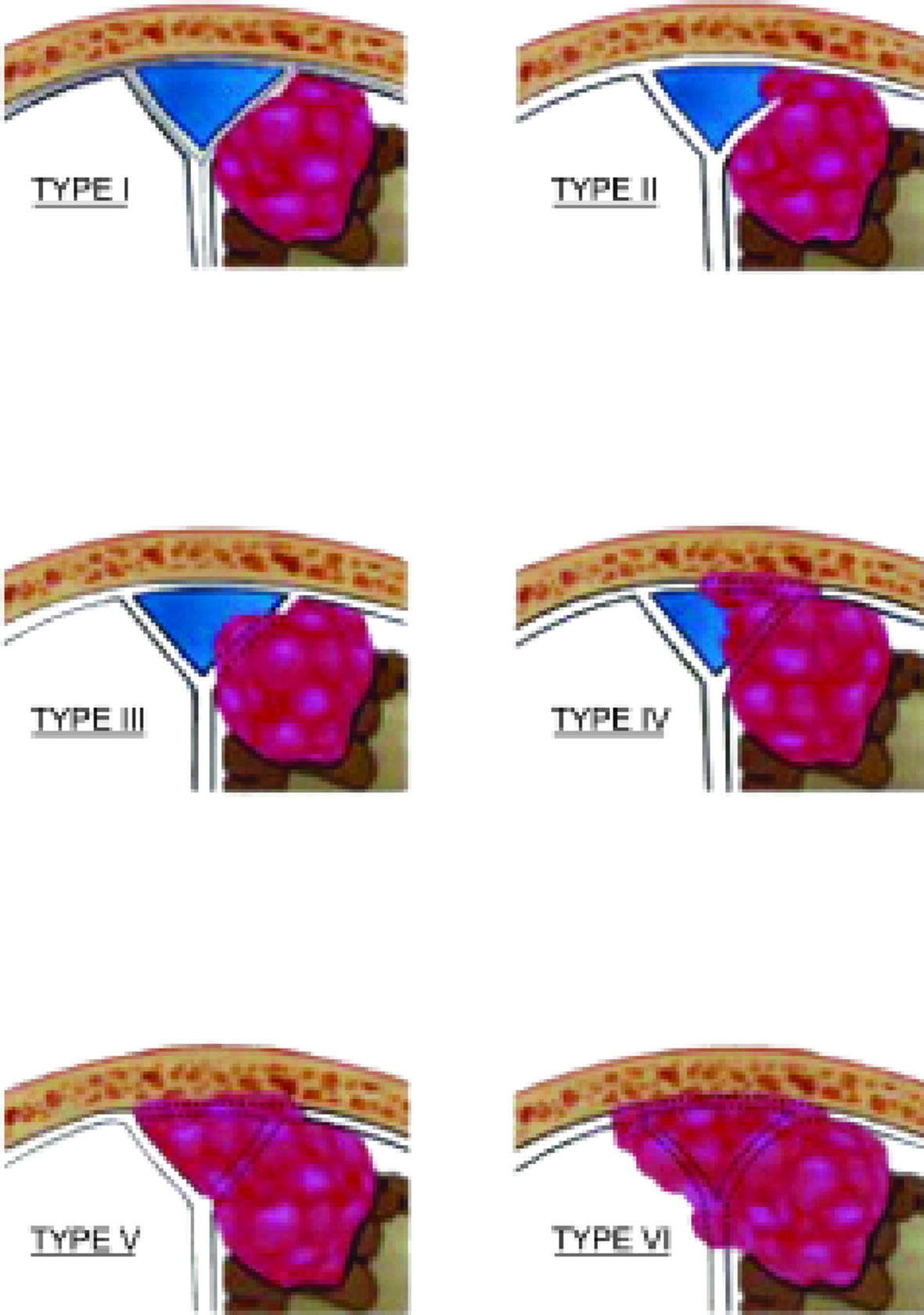
Fig. 3. Schematic representation of types of invasion of the SSS. Type I – the meningioma is adjacent to the outer wall of the sinus; type II – invasion into the lateral corner of the SSS; type III – invasion into the ipsilateral wall of the sinus; type IV – invasion into both the ipsilateral wall and roof of the sinus; type V – total occlusion of the sinus, but the contralateral wall remains intact; type VI – total invasion of the SSS involving all walls of the sinus
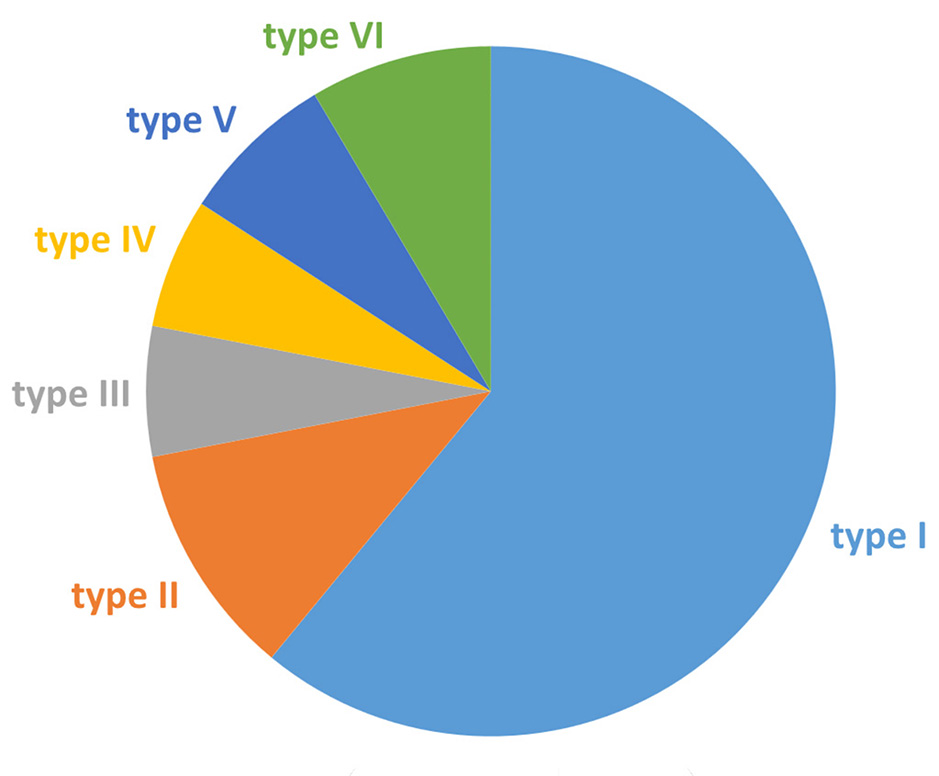
Fig. 4. Distribution of SSS meningiomas according to the M.P. Sindou and J.E. Alvernia classification
The majority (71%) of SSS meningiomas were located in the middle third of the sinus, corresponding to the area between the coronal and lambdoid sutures, aligning with data from the literature (45.0‒70.5%). Meningiomas of the anterior third of the sinus (from the crista galli to the coronal suture) accounted for 22% (18 patients), while those of the posterior third of the sinus made up 7% (6 patients) (Fig. 5).
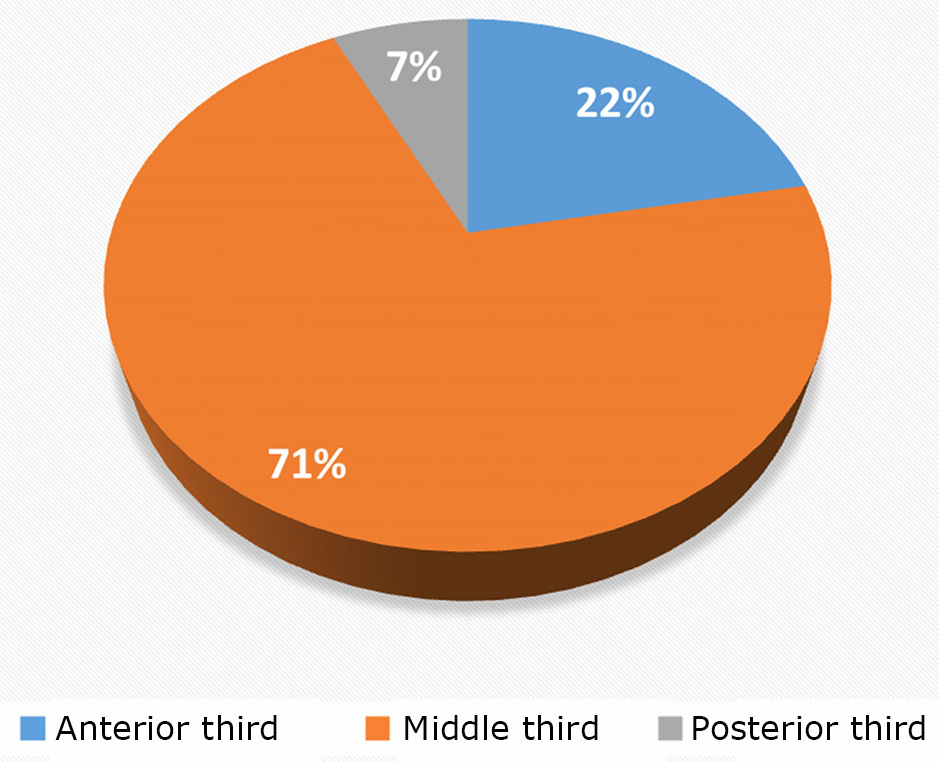
Fig. 5. Distribution of patients based on the "growth zone" of the tumor concerning the SSS
The clinical symptoms in patients with SSS meningiomas included seizures (29.3%), headaches (89.0%), and varying degrees of hemiparesis (37.8%).
Surgical Treatment
Patients were positioned laterally in a park-bench position or supine with head fixation using a three-point Mayfield clamp. A horseshoe-shaped skin incision, based at the SSS, was made with particular attention to preserving the integrity of the periosteum for subsequent dura mater reconstruction. Craniotomy was performed, typically using two burr holes placed in the SSS projection based on MRI and MSCT angiography data. Bone flap craniotomy was extended across the midline. The next step involved incision and possible resection of the affected dura mater, devascularization of the tumor from the falx cerebri and SSS, piecemeal tumor resection, and removal. In 68 cases (81%), a conservative approach with coagulation of the falx cerebri was adopted, while in 16 cases (19%), an aggressive approach involving excision of the affected segment of the falx cerebri was employed, with SSS resection in 12 (75%) of these cases. The latter was predominantly observed in patients with type V and VI invasion as per M.P. Sindou and J.E. Alvernia's classification. All 12 patients underwent the SSS reconstruction of the dura mater using the periosteum, muscle, or "TachoComb" (Takeda, Austria). Dural defect repair was conducted with the periosteum or artificial dura to prevent postoperative cerebrospinal fluid (CSF) leakage.
A total of 84 surgical procedures were performed on 82 patients, including 71 (84.5%) primary surgeries and 13 (15.5%) reoperations. Among the 13 reoperations, invasion into the SSS was newly detected in 7 cases based on neuroimaging and confirmed intraoperatively (p=0.001).
Two reoperations were necessitated by epidural hematoma due to low molecular weight heparin administration for SSS thrombosis prevention. Of the 68 subtotal tumor resections (Simpson III) [7], caused by residual tumor in the sinus cavity, 21 (30.9%) patients were elderly (>75 years), and in 42 (61.8%), the tumor involved the middle third of the SSS with venous drainage involvement.
Partial SSS thrombosis was diagnosed in 5 asymptomatic cases (6%) on postoperative MSCT angiography, correlating with type V-VI invasion according to M.P. Sindou and J.E. Alvernia (p=0.001).
Postoperative CSF leakage occurred in 3 (3.6%) cases, which were managed conservatively with lumbar drainage for 4–5 days and anti-edema therapy without requiring further surgery.
In the postoperative period, 41 (50.0%) patients experienced varying degrees of hemiparesis. Neurological deficits worsened in 10 patients due to surgery but fully regressed within 3–6 months in 28 cases.
Seizure control (Engel I: seizure-free) was achieved in 22 (91.7%) out of 24 patients with preoperative epilepsy after 3–6 months of follow-up [8].
Within 3 months after surgery, 27 (32.9%) patients complained of recurrent headache.
Persistent tumor growth was detected in 4 patients (4.9%) between 2.5–6.0 years postoperatively. Histological analysis confirmed “anaplastic meningioma Grade III” in 3 cases and “atypical meningioma Grade II” in 1 case [9]. All patients were referred for radiosurgical treatment, achieving remission consistent with the literature (p<0.05) [10].
Conclusions
Meningiomas originating from the arachnoid membrane represent a significant portion of primary intracranial tumors with varying degrees of venous sinus invasion. Surgical planning for SSS-invasive meningiomas should consider radiological invasion classifications to guide treatment strategy.
Preoperative MRI with intravenous contrast and MSCT angiography allow for identifying collateral blood flow and determining the degree of sinus invasion.
Recognizing angiographic vascularization features of meningiomas aids in surgical strategy. A clear understanding of the topography of afferent tumour vessels facilitates intraoperative identification and timely blockage of them to prevent excessive bleeding which complicates the performance of the planned volume of surgical intervention.
Postoperative monitoring of neurological status and complications remains critical for patients undergoing surgical treatment of SSS meningiomas.
Disclosure
Conflict of Interest
The authors declare no conflicts of interest.
Ethical Standards
All procedures performed on patients during the study adhered to the ethical standards of the institutional and national ethics committees, as well as the 1964 Helsinki Declaration and its subsequent amendments or comparable ethical standards.
Informed Consent
Informed consent was obtained from all patients.
Funding
The study was not supported by any sponsorship.
References
1. Aghi M, Barker Ii FG. Benign adult brain tumors: an evidence-based medicine review. Prog Neurol Surg. 2006;19:80-96. https://doi.org/10.1159/000095184
2. Schmutzer M, Skrap B, Thorsteinsdottir J, Fürweger C, Muacevic A, Schichor C. Meningioma involving the superior sagittal sinus: long-term outcome after robotic radiosurgery in primary and recurrent situation. Front Oncol. 2023 Jul 11;13:1206059. https://doi.org/10.3389/fonc.2023.1206059
3. Gatterbauer B, Gevsek S, Höftberger R, Lütgendorf-Caucig C, Ertl A, Mallouhi A, Kitz K, Knosp E, Frischer JM. Multimodal treatment of parasagittal meningiomas: a single-center experience. J Neurosurg. 2017 Dec;127(6):1249-1256. https://doi.org/10.3171/2016.9.JNS161859
4. Gagliardi F, De Domenico P, Snider S, Pompeo E, Roncelli F, Barzaghi LR, Acerno S, Mortini P. Efficacy of radiotherapy and stereotactic radiosurgery as adjuvant or salvage treatment in atypical and anaplastic (WHO grade II and III) meningiomas: a systematic review and meta-analysis. Neurosurg Rev. 2023 Mar 17;46(1):71. https://doi.org/10.1007/s10143-023-01969-7
5. Gravbrot N, Rock CB, Weil CR, Rock CB, Burt LM, DeCesaris CM, Jensen RL, Shrieve DC, Cannon DM. Gross Tumor and Intracranial Control Benefits with Fractionated Radiotherapy Compared with Stereotactic Radiosurgery for Patients with WHO Grade 2 Meningioma. World Neurosurg. 2024 Aug;188:e259-e266. https://doi.org/10.1016/j.wneu.2024.05.093
6. Sindou MP, Alvernia JE. Results of attempted radical tumor removal and venous repair in 100 consecutive meningiomas involving the major dural sinuses. J Neurosurg. 2006 Oct;105(4):514-25. https://doi.org/10.3171/jns.2006.105.4.514
7. Simon M, Gousias K. Grading meningioma resections: the Simpson classification and beyond. Acta Neurochir (Wien). 2024 Jan 23;166(1):28. https://doi.org/10.1007/s00701-024-05910-9
8. Fisher RS, van Emde Boas W, Blume W, Elger C, Genton P, Lee P, Engel J Jr. Epileptic seizures and epilepsy: definitions proposed by the International League Against Epilepsy (ILAE) and the International Bureau for Epilepsy (IBE). Epilepsia. 2005 Apr;46(4):470-2. https://doi.org/10.1111/j.0013-9580.2005.66104.x
9. Torp SH, Solheim O, Skjulsvik AJ. The WHO 2021 Classification of Central Nervous System tumours: a practical update on what neurosurgeons need to know-a minireview. Acta Neurochir (Wien). 2022 Sep;164(9):2453-2464. https://doi.org/10.1007/s00701-022-05301-y
10. Lubgan D, Rutzner S, Lambrecht U, Rössler K, Buchfelder M, Eyüpoglu I, Fietkau R, Semrau S. Stereotactic radiotherapy as primary definitive or postoperative treatment of intracranial meningioma of WHO grade II and III leads to better disease control than stereotactic radiotherapy of recurrent meningioma. J Neurooncol. 2017 Sep;134(2):407-416. https://doi.org/10.1007/s11060-017-2540-7