Original article
Ukrainian Neurosurgical Journal. 2025;31(1):16-22
https://doi.org/10.25305/unj.311770
1 Department of Neurotrauma, Romodanov Neurosurgery Institute, Kyiv, Ukraine
2 Department of Histology and Embryology, Bogomolets National Medical University, Kyiv, Ukraine
3 Neuropathomorphology Department, Romodanov Neurosurgery Institute, Kyiv, Ukraine
4 Restorative Neurosurgery Department, Romodanov Neurosurgery Institute, Kyiv, Ukraine
5 Neuroimmunology Department, Romodanov Neurosurgery Institute, Kyiv, Ukraine
6 Department of Miniinvasive and Laser Spinal Neurosurgery, Romodanov Neurosurgery Institute, Kyiv, Ukraine
Received: 18 September 2024
Accepted: 28 November 2024
Address for correspondence:
Andrii B. Panteleichuk, Department of Neurotrauma, Romodanov Neurosurgery Institute, 32 Platona Mayborody st., Kyiv, Ukraine, 04050, e-mail: basirovich@ukr.net
Background. Treatment of glioblastoma is an extremely important problem of neuro-oncology. Despite the presence of modern medical and technological developments, there is no significant progress in solving it today. Valproic acid (VPA) is an antiepileptic drug with proven efficacy in epilepsy, and potential oncostatic effects of VPA in the treatment of brain tumors are considered. This study examined the effect of VPA on the growth of rat C6 glioma in vivo
Methods. After experimental glioma modeling in rats, valproic acid injections were performed intraperitoneally. Survival of rats was studied according to the Kaplan-Meier curve and tumors were examined histologically. Conclusions about proliferative activity were made based on the determination of the index of Ki67-positive cells
Results. Valproic acid significantly increased the median survival rate of rats with gliomas from 11 to 13 days (p=0,05) and significantly decreased the proliferative activity of glioma cells (3.53±0.96, Me=3.08 vs 2.17±0.38, Me=2.11, p=0,05)
Conclusions. These findings indicate that valproic acid inhibits the growth of glioma cells in vivo, which can be considered as a promising tool in the complex therapy of gliomas in clinical practice and is a perspective for further research
Key words: rat C6 glioma; survival; valproic acid (VPA); proliferation
Introduction
Glioblastoma is a tumor that originates from glial cells of the central nervous system, characterized by aggressive malignant growth, a high rate of recurrence and high mortality rate of patients [1].
Increasing the survival rates and improving the quality of life for patients with glioblastoma is an extremely urgent problem of neurosurgery, since the average life expectancy of such patients is 14.6 months, and the five-year survival rate in developed countries does not exceed 6.3%. Therefore, scientific research and optimization of both surgical techniques and adjuvant treatment methods remain highly relevant: over the last 25 years the life expectancy of patients has increased with glioblastoma by only 3.3 months [2, 3]. Survival rates are one of the most significant criterium in oncology. They are used for the most adequate evaluation of the effectiveness of various treatment methods in clinical studies, evaluation of the medical care organization effectiveness for the population in oncoepidemiological population studies [4] in experimental medical and biomedical research [5]
Kaplan-Meier (KM) survival analysis is a non-parametric estimate of the survival function that is commonly used to describe the survival of a study population and to compare two study populations [6]. This indicator is a reliable and convenient tool for comparing the life expectancy of patients (or experimental animals) when investigating new treatment methods compared to a control group (standard treatment). Thus, by comparing two or more survival curves, you can get a visual conclusion about the effectiveness of experimental treatment. The essence of such a comparison is to test null hypothesis - the assumption that survival in the two groups does not differ and the studied factor (treatment method) does not affect the studied value (survival rates). If there is a significant difference in the survival of the two studied groups, null hypothesis is rejected, which will indicate the effectiveness of a new or experimental method of treatment [7].
Animal models of tumors are valuable for studying the biological features of tumors and identifying agents with a potential oncostatic effect. The C6 glioma cell line was isolated from Wistar-Furthi rats, and is of astrocytic origin is both a well-characterized and most widely used glioma model, given its high malignancy and aggressiveness [5]. Rat glioma cells express a similar profile of human glioma markers (PDGFβ, IGF-1, EGFR, Erb3/Her3) [8]. C6 glioma is a model of glioma cell proliferation and migration and is used to evaluate the potential antitumor growth of new drugs [5, 8].
The use of antiepileptic drugs in clinical practice (in the complex therapy of gliomas) is natural, since the frequency of symptomatic epilepsy can reach 100%, and in particular in glioblastoma - up to 60% [9]. Valproic acid, an antiepileptic drug from the valproate group, is primarily indicated for various forms of epilepsy, migraine management, bipolar disorders, etc. It also exhibits antipsychotic effects [10] and acts as a radiosensitizer in glioma treatment [11]. The realization of the clinical effects of VPA is carried out through its various mechanisms: blockade of sodium and calcium channels of cell membranes, as well as an increase in the concentration of GABA in brain tissues [12]. The oncostatic effect of VPA has been described in many types of tumors: gastric and breast cancers, etc., research data is still ongoing [13, 14], and is relatively new. Regarding glioblastoma, this effect of VPA has been known since the early 2000s, when the effect of histone deacetylase inhibition was discovered, which led to a decrease in mitotic activity in tumor cells and, as a result, to a decrease in its progression [6, 15, 16]. Data from experimental studies in vivo and in vitro on inhibition of glioblastoma growth under the influence of VPA show encouraging results [17, 18, 19], but the results of clinical use are quite contradictory and ambiguous: it is reported about a positive experience of using VPA in glioblastomas and anaplastic astrocytomas [11, 10], especially in children and young people, while it is completely ineffective for neuro-oncology patients [20].
Thus, the search for new treatment methods and the potential effect of VPA on glioma remains relevant. In this study we focused on investigating the effects of valproic acid on survival of laboratory animals using the well-characterized rat brain C6 glioma cell line, aiming to establish the potential impact of valproic acid on the proliferative activity of glioma cells in vivo.
Aim of the study: To determine the effect of valproic acid on the proliferative activity of C6 rat glioma cells based on the analysis of Ki67 indicators and the survival of animals with glioma. To determine the prospects and feasibility of further studies of the oncostatic effect of VPA in the treatment of glial tumors, based on the results of this experiment and literature data.
Materials and methods
C6 glioma model
White outbred male rats weighing 100±25 g, with an average age of 2 months, were selected for the study. The animals were kept in the vivarium under the conditions of a natural circadian light cycle. They were fed a combined balanced diet ad libitum and with free access to water. The control group was 10 animals and the experimental group was 14 animals. To transplant glioma cells from a sick animal, a tumor was removed from which a cell suspension was prepared. After counting the cells in the Horyaev chamber, all animals were implanted with C6 rat glioma cells (2×106 cells in 0.1 ml) in the right temporal area to a depth of 4 mm, as described [21].
This study was conducted in strict accordance with the recommendations of the National Institutes of Health’s Guide for the Care and Use of Laboratory Animals. The research protocol with animal experimentation was approved by the Scientific Ethics Committee (Protocol Number: 17/2022). All surgery was performed under general anesthesia and every effort was made to minimize suffering. The study was conducted on two groups of animals with glioma modeling: 1 – control; 2 - experimental, which was given VPA treatment.
Surgical procedure
Manipulations with animals were carried out under general anesthesia - intraperitoneal injection of thiopental sodium 50-60 mg/kg of animal weight. The injection site was previously disinfected with a 5% iodine solution.
All surgical procedures were carried out using sterile instruments in operation room. Prior to surgery, animals were fixed in stereotactic device. Skull operative area, was decontaminated by betadine solution with a cotton swab. Coronal suture was exposed through midline scalp incision. Cranial burrhole was made 4 mm to the right of midline and 1 mm anterior to the coronal suture by a mini-drill. A 26-gauge stainless steel guide cannula was stereotactically implanted through this hole to a depth of 4 mm and the suspension of C6 rat glioma cells was injected (2×106 cells in 0.1 ml) in the right temporal area. The skin was closed using 7–0 (ETICON) atraumatic sutures.
The animals were observed daily and their lifespan was recorded. On the 5th day, one animal in the control group and on the 6th day one animal in the experimental group dropped out due to death, neither death was related to the tumor; in addition, on the 10th day, 6 animals were removed from both groups to study and compare the morphological features of the tumor. These animals were not taken into account in the calculation of survival rates due to identical elimination dynamics in the groups. Thus, the number of animals in the control and experimental groups was 10 and 14, respectively (see Table 1).
Table 1. Number of animals
|
Group |
Animals, N |
|
Control |
10 |
|
Experimental |
14 |
|
Overall |
24 |
The preparation of valproic acid in the form of a solution of 100 mg/ml was administered intraperitoneally at a dose of 300 mg/kg of the animal's body once daily throughout the experiment. This dose was selected based on its safety profile and its lack of undesirable biological reactions [22]. Animals were removed from the experiment by injecting a lethal dose of sodium thiopental intraperitoneally.
Histological examination
At 10th day of experiment, rat brains were fixed in a 10% formalin solution (pH 7.4, 4°C, 24 hours). Tumors were isolated from each brain, dehydrated in isopropanol and sealed in paraplast (Leica Surgipath Paraplast Regular). Sections with a thickness of 4 μm were obtained on a Thermo Microm HM 360 microtome. Sections after deparaffinization were stained with Sirius Red and Weigert's hematoxylin, hematoxylon and eosin to study tumor morphology. Proliferative activity was assessed by immunohistochemical method based on the analysis of immunopositive reactions to Ki67. (https://www.abcam.com/en-us/products/primary-antibodies/ki67-antibody-sp6-ab16667#tab=datasheet) A monoclonal antibody against Ki67 (Abcam, USA, Cat.number: ab 16667) was used. Primary antibodies were used at a dilution of 1:200. Reaction products were visualized using a diaminobenzidine-based detection system (EnVision FLEX; Dako, Glostrup, Denmark). Sections were incubated with antibodies at a temperature of +24°C (with primary and secondary antibodies for 20 and 10 min, respectively). Cell nuclei were stained with hematoxylin Gill I. The obtained micropreparations were examined in an Olympus BX51 microscope, magnification ×200, ×400. Proliferative activity was evaluated as the relative number of Ki67-positive cells in 10 fields of view for each tumor sample, as the number of Ki67 positive cells to the total number of cells in the region of interest.
Statistical analysis
Data processing was carried out using the statistical software package Statistica 10.0 (StatSoft Inc., USA). Survival rates were standardized, entered into the database and subjected to statistical processing using descriptive and non-parametric statistical methods. Survival analysis was performed using the Kaplan-Meier method. Pearson's χ2 test was used to determine the difference between the survival of animals in the study and control groups. When comparing survival in different groups, the risk ratio with a 95% confidence interval (CI) was used. The Mann-Whitney test was used to analyze the difference in the values of proliferative activity between groups. The results were considered statistically significant provided that the level of statistical significance (p) was <0.05. Spearman correlation coefficient was used to analyze the correlation between Ki-67 index and tumor mass. The results are given in the discussion.
Results
Survival analysis
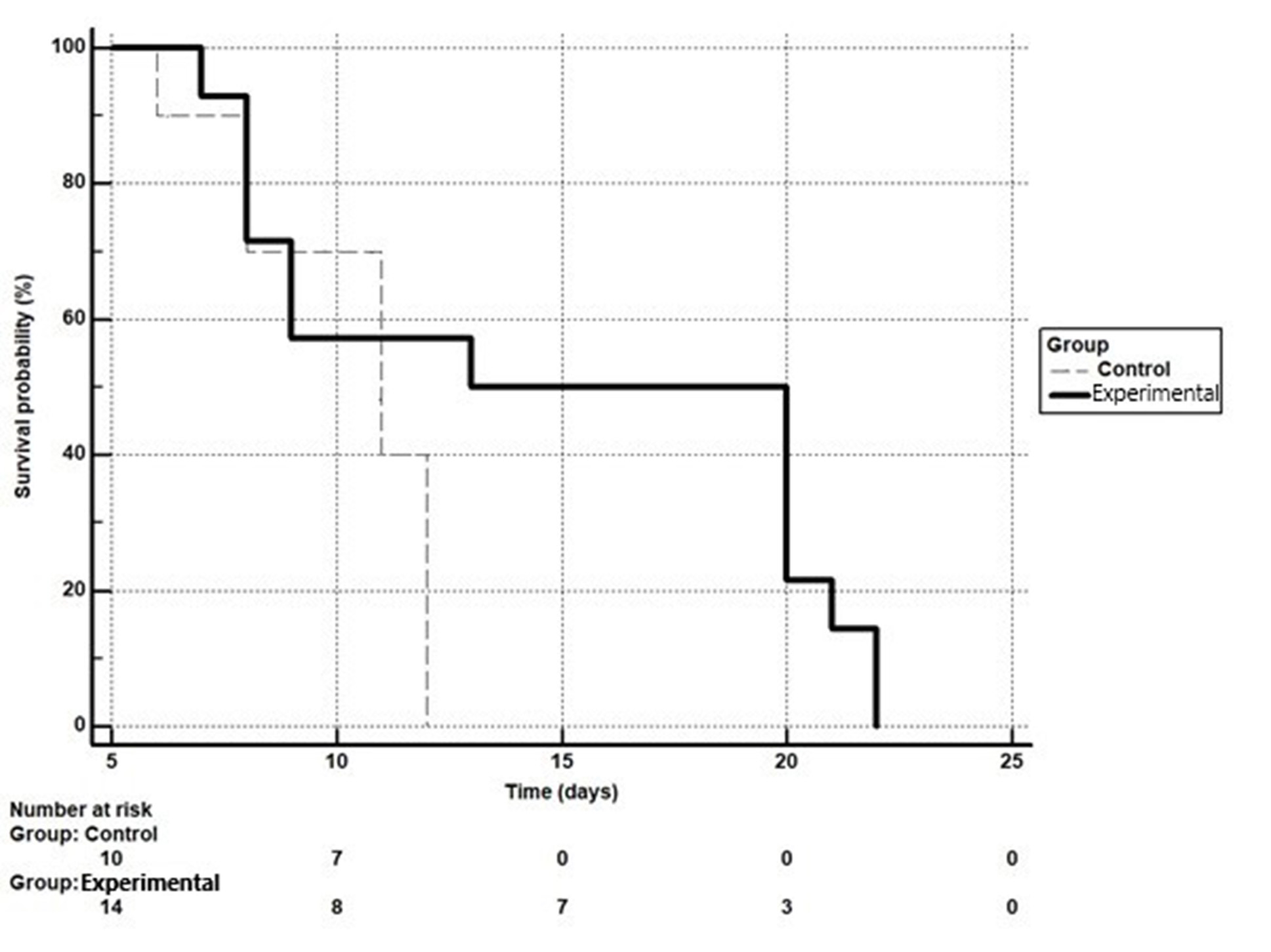
In the control group, one animal died on the 6th day, two animals on the 8th day, three animals on the 11th day, and four animals on the 12th day. In the experimental group: on the 7th day – one animal, on the 8th day – three animals, on the 9th day – two animals, on the 13th day – one animal, on the 20th day – four animals, on the 21st day – one animal and on the 22nd day – two animals. Generalized data are shown in Fig. 1. The results of statistical processing of the given data with survival rates are shown in Table 2.

Fig. 1. Kaplan-Meier curve of survival of rats of the control and experimental groups. The difference between groups according to chi-square results is statistically significant (p=0.04)
Table 2. Survival rate of animals
|
Group |
Mean survival (days) |
SE |
Confidence interval for the mean 95% |
Median survival (days) |
Confidence interval for the median 95% |
|
Control |
10,300 |
0,684 |
8,959 to 11,641 |
11,000 |
8,000 to 12,000 |
|
Experimental |
14,786 |
1,691 |
11,472 to 18,100 |
13,000 |
8,000 to 20,000 |
Thus, the median survival in the control group was 11 days, in the experimental group 13 days, which is demonstrated by the Kaplan-Meier curves in Fig. 1.
For the experimental group versus the control group: hazard ratio (HR)=0.51 (0.21-1.26), p=0.0448 – relative risk reduced by 49%. It is defined as (1-0.51)*100. For the control group versus the experimental group: HR=1.96 (0.79-4.84), p=0.0448 – relative risk increased by 1.96 times (Table 3).
Table 3. Hazard ratios of survival with 95% confidence interval
|
Group |
Hazard ratio of survival (HR) and 95%CI |
|
Experimental vs Control |
0,51 (0,21 to 1,26) |
|
Control vs Experimental |
1,96 (0,79 to 4,84) |
The risk of death in the control group compared to the experimental group is 1.96 times higher (HR=1.96), but the risk of death in the experimental group compared to the control group is reduced by 49% (HR=0.51).
Tumor morphology
10 days after implantation of C6 glioma cells into the brain of rats, intracerebral tumors were detected. The tumors had a small volume and clear borders. This proved that the glioma model was successfully reproduced. The average weight of tumors was 254.1 ± 38.67 mg in the control group and 308.3 ± 55.8 in the experimental group (p>0.05). Glioma tissues in both the control and experimental groups were characterized by moderate nuclear atypia (nuclear pleomorphism) and not high mitotic activity. It was also found that a certain proportion of tumor cells in both groups had cytoplasmic vacuolation; this feature was slightly more prevalent in the experimental group. According to the results of staining with hematoxylin and eosin, Sirius Red, tumors grew intracerebral, glioma cells occasionally extended into the dura mater which is rich in collagen. The peripheral zone of the tumors had less vascularization than in the central part of the tumor. Areas of sparse necrosis were observed in the central part of the tumor contained only single Ki67-positive cells (Fig. 2). The Ki67 index in the control group was 3.5%, and in the experimental group it was 2.1% (Fig. 3, Table 4). According to the results of the assessment of the one-sided Mann-Whitney test, the difference was probable at p=0.01, and for two-sided p=0.02. Thus, the study results revealed a slight but statistically significant difference between the values of the proliferative activity index in the comparison groups. At the same time, a negative correlation was found between the mass of tumors and the average value of the proliferative index in the general sample of the experiment (r=-0.61, p=0.04), while for the control and experimental groups, the correlation analysis did not show a probable dependence (r =-0.71, p=0.11 vs r=-0.49, p=0.33).
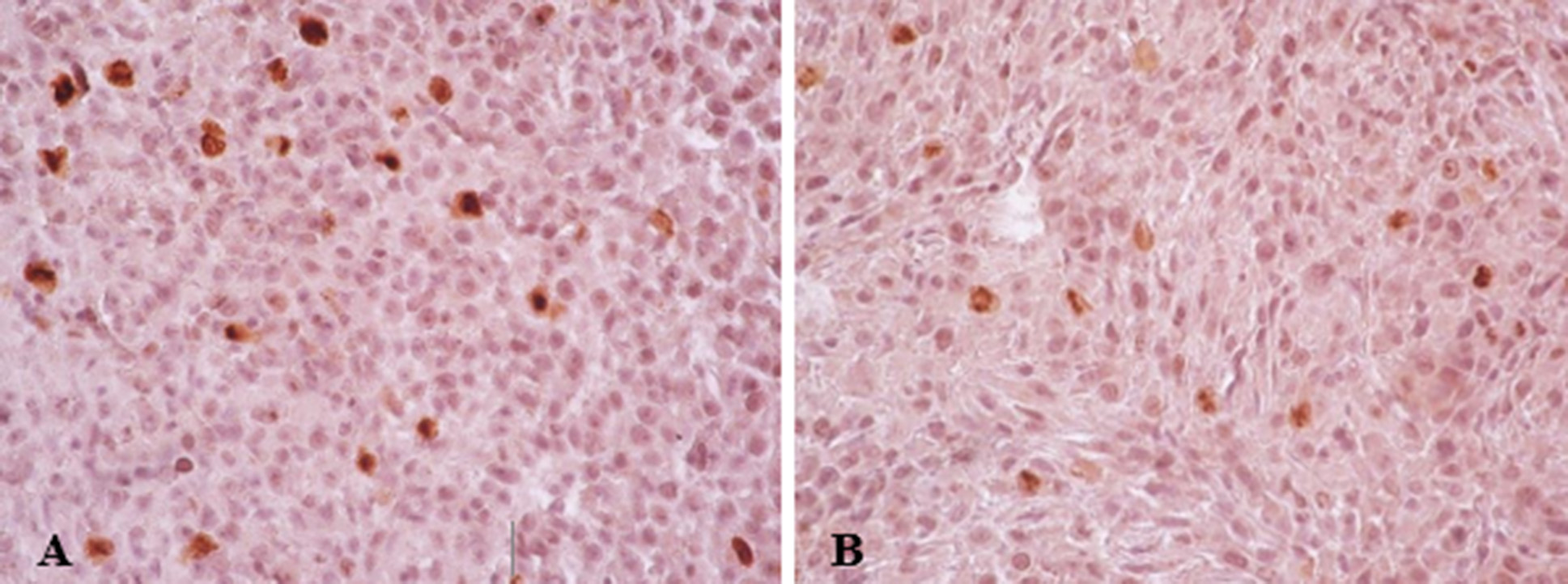
Fig. 2. Micrographs of C6 glioma tumor tissues, Ki67-positive cells (brown). Control group (A) and the experimental group with valproic acid (B). Immunohistochemical detection of Ki67. Rev. 40, approx. 10
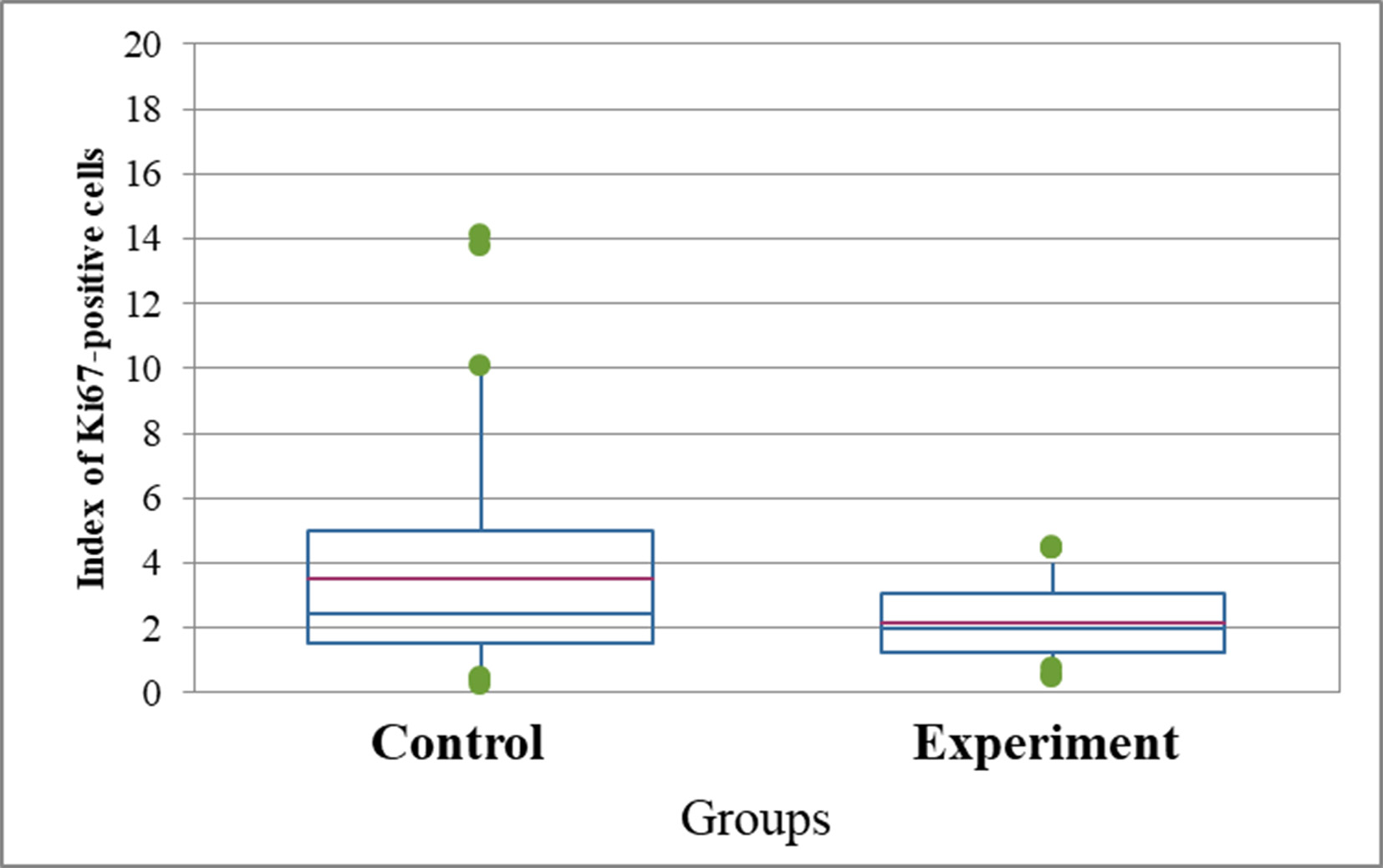
Fig. 3. Index of Ki67-positive C6 cells in glioma tumors according to the results of Ki67 immunohistochemical detection (%). For the one-sided Mann-Whitney test p=0.01, for two-sided p=0.02
Table 4. Index of Ki67-positive C6 cells in tumor tissues of control and experimental groups of rats on day 10 (n=24)
|
Group |
Mean |
SEM |
Minimum |
Q1 |
Median |
Q3 |
Maximum |
|
Control |
3,53 |
0,96 |
1,29 |
1,45 |
3,08 |
5,51 |
6,48 |
|
Experimental |
2,17 |
0,38 |
1,11 |
1,42 |
2,11 |
3,03 |
3,18 |
Notes. Mean - arithmetic mean value of the data in the group; Median - the average value from the main data array in the group; Q1 and Q3 are the interquartile range in the data set. All these indicators are used when conducting data analysis using the Mann-Whitney method, when the samples in both groups differ from the Gaussian distribution, making parametric methods inapplicable.
Discussion
The rat C6 glioma model is widely used as an animal model in human glioblastoma research because it is similar in terms of growth and biological behavior to malignant brain gliomas [5, 23].
The results of in vivo research on the C6 glioma model in rats indicate a significant difference in both survival rates and proliferative activity (Ki67) in glioma-bearing rats without treatment comparing to glioma-bearing rats with VPA treatment, which correlates with literature data on this issue. In our opinion, a certain paradoxical situation has arisen regarding the effectiveness of VPA in the treatment of glial tumors: on the one hand, experimental data both in vitro and in vivo (and our own experience) convincingly testify to the oncostatic effect of valproic acid on glial tumors; on the other hand, there is no convincing clinical data on such effectiveness. This raises the question of prospects and possible directions of further research: possible ways to overcome the obvious difference between experimental data and clinical results can be implemented as follows:
1) increasing the dose of VPA to the maximum possible level for each specific patient [23] while exploring strategies to mitigate its toxic effect of VPA on the patient's body; for example, the use of various carbon nanomaterials (nanotubes, fullerenes, etc.) is promising for reducing toxicity [24];
2) combination with other medicinal products to potentiate their effect; for example, taking VPA in combination with temozolomide increased the median survival of patients with glioblastoma by 8 weeks, compared with the group where patients received temozlomide montherapy [10, 22];
3) combination with other treatment methods, such as radiosurgery, radiochemotherapy and others [25, 11];
4) development of VPA-based drugs: separate experimental studies demonstrate that various VPA derivatives have different anticonvulsant, antitumor and histone deacetylase inhibition properties compared to VPA itself [26, 27, 28];
5) advancing drug delivery methods to target cells, for example, the use of nanoparticles containing an oncostatic agent (doxorubicin) has demonstrated a 40% cure rate in laboratory animals [29].
Another unsolved question that arises from the results of this work is the absence of a positive correlation between tumor mass and Ki67 as an indicator of proliferative activity of tumor C6 cells. In other studies in the C6 rat model, such a strong relationship was demonstrated between Ki67 values and mean tumor size measured by spectral CT [30] and studying the mechanisms of tumor cell death.
This issue requires further analysis and research by other methods, in particular, the detection of cellular markers of cell death.
Therefore, further studies of the VPA action in neuro-oncology are essential. Evaluating the impact of VPA on malignant cells suggests several possible mechanisms for its oncostatic action: a direct effect on the mitotic cycle by inhibiting histone deacetylase and an indirect one - through the inhibition of angiogenesis, activation of aging mechanisms and death of tumor cells. Cell death is typically caused by apoptosis or necrosis. However, an alternative scenario may occur - autophagy. It is already known that VPA is able to initiate a moderate apoptotic response by preferential activation of the mitochondrial pathway in cancer cells [31] and induce cell death through the autophagy pathway [32]. Microscopic differences in autophagy in cells include the detection of large vacuoles in the cytoplasm and specifically in the presence of VPA. In our study, vacuolization was not quantified as the primary focus was mainly aimed at an integral assessment of the oncostatic effect - that is, overall survival. However, the weak signs of vacuoles in the cytoplasm of cells in the VPA group suggests that autophagic cell death induced by VPA may be involved in the tumor growth inhibition. VPA was also found to disrupt blood vessel formation by reducing eNOS expression [33]. In general, given the diversity of VPA effects on biological systems, at the level of macromolecules, cells, tumor tissue, and the organism as a whole, the molecular mechanisms of the oncostatic action of VPA require further research.
Conclusions
Disclosure
Funding
No funding was received for this research
Conflicts of interest/Competing interests
All authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers’ bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent-licensing arrangements), or non-financial interest (such as personal or professional relationships, affiliations, knowledge or beliefs) in the subject matter or materials discussed in this manuscript.
Availability of data and material
Not applicable
Code availability
Not applicable
Ethics approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. The work was approved by the Bioethics Committee No. 43 dated 04.24.23
Consent to participate
Not applicable
Consent for publication
Not applicable
References
1. Ren Z, Wen J, Mo Y, Zhang P, Chen H, Wen J. A systematic review and meta-analysis of fluorescent-guided resection and therapy-based photodynamics on the survival of patients with glioma. Lasers Med Sci. 2022 Mar;37(2):789-797. https://doi.org/10.1007/s10103-021-03426-7
2. Sirko AG, Dzyak LA, Balashova OI, Berdova TL, Donchenko GN, Skljar NV, Shestakova NN, Romanukha DN. Prediction of treatment results of low-grade gliomas of the cerebral hemispheres. Medicni perspektivi. 2017;22(3):52-9. https://doi.org/10.26641/2307-0404.2017.3.111926
3. Wang LM, Englander ZK, Miller ML, Bruce JN. Malignant Glioma. Adv Exp Med Biol. 2023;1405:1-30. https://doi.org/10.1007/978-3-031-23705-8_1
4. Schepotin IB, Fedorenko ZP, Gulak LO, Ryzhov AY, Gorokh YL, Sumkina OV, Kutsenko LB. Survival rate as a measure for evaluation of cancer care for population. Clinical Oncology. 2013;4(12):1-4. https://www.clinicaloncology.com.ua/en/article/26040/vikoristannya-pokaznika-vizhivanosti-yak-kriteriyu-ocinki-yakosti-onkologichnoi-dopomogi-naselennyu-2
5. Giakoumettis D, Kritis A, Foroglou N. C6 cell line: the gold standard in glioma research. Hippokratia. 2018 Jul-Sep;22(3):105-112.
6. Göttlicher M. Valproic acid: an old drug newly discovered as inhibitor of histone deacetylases. Ann Hematol. 2004;83 Suppl 1:S91-2. https://doi.org/10.1007/s00277-004-0850-2
7. Holubova H. [Kaplan-Meyer survival curves: simulation technique]. Scientific Bulletin of the National Academy of Statistics, Accounting and Audit. 2021 Dec 21(3-4):15-22. Ukrainian. https://doi.org/10.31767/nasoa.3-4-2021.02
8. Śledzińska P, Bebyn MG, Furtak J, Kowalewski J, Lewandowska MA. Prognostic and Predictive Biomarkers in Gliomas. Int J Mol Sci. 2021 Sep 26;22(19):10373. https://doi.org/10.3390/ijms221910373
9. Hauff NS, Storstein A. Seizure Management and Prophylaxis Considerations in Patients with Brain Tumors. Curr Oncol Rep. 2023 Jul;25(7):787-792. https://doi.org/10.1007/s11912-023-01410-8
10. Kamarudin MNA, Parhar I. Emerging therapeutic potential of anti-psychotic drugs in the management of human glioma: A comprehensive review. Oncotarget. 2019 Jun 11;10(39):3952-3977. https://doi.org/10.18632/oncotarget.26994
11. Sullivan JK, Fahey PP, Agho KE, Hurley SP, Feng Z, Day RO, Lim D. Valproic acid as a radio-sensitizer in glioma: A systematic review and meta-analysis. Neurooncol Pract. 2022 Sep 30;10(1):13-23. https://doi.org/10.1093/nop/npac078
12. Tseng JH, Chen CY, Chen PC, Hsiao SH, Fan CC, Liang YC, Chen CP. Valproic acid inhibits glioblastoma multiforme cell growth via paraoxonase 2 expression. Oncotarget. 2017 Feb 28;8(9):14666-14679. https://doi.org/10.18632/oncotarget.14716
13. Pak O, Kosianova A, Zaitsev S, Sharma A, Sharma H, Bryukhovetskiy I. Valproic Acid and Celecoxib Enhance the Effect of Temozolomide on Glioblastoma Cells. CNS Neurol Disord Drug Targets. 2024 Oct 18. https://doi.org/10.2174/0118715273330268241008220702
14. Han W, Guan W. Valproic Acid: A Promising Therapeutic Agent in Glioma Treatment. Front Oncol. 2021 Sep 10;11:687362. https://doi.org/10.3389/fonc.2021.687362
15. Santos DS, Rocha MA, Mello MLS. Epigenetic studies in insects and the valproic acid perspective. Braz J Biol. 2022 Apr 8;84:e256045. https://doi.org/10.1590/1519-6984.256045
16. Drzewiecka M, Gajos-Michniewicz A, Hoser G, Jaśniak D, Barszczewska-Pietraszek G, Sitarek P, Czarny P, Piekarski J, Radek M, Czyż M, Skorski T, Śliwiński T. Histone Deacetylases (HDAC) Inhibitor-Valproic Acid Sensitizes Human Melanoma Cells to Dacarbazine and PARP Inhibitor. Genes (Basel). 2023 Jun 20;14(6):1295. https://doi.org/10.3390/genes14061295
17. Fiorentino F, Fabbrizi E, Raucci A, Noce B, Fioravanti R, Valente S, Paolini C, De Maria R, Steinkühler C, Gallinari P, Rotili D, Mai A. Uracil- and Pyridine-Containing HDAC Inhibitors Displayed Cytotoxicity in Colorectal and Glioblastoma Cancer Stem Cells. ChemMedChem. 2024 Jul 2;19(13):e202300655. https://doi.org/10.1002/cmdc.202300655
18. Berendsen S, Frijlink E, Kroonen J, Spliet WGM, van Hecke W, Seute T, Snijders TJ, Robe PA. Effects of valproic acid on histone deacetylase inhibition in vitro and in glioblastoma patient samples. Neurooncol Adv. 2019 Nov 12;1(1):vdz025. https://doi.org/10.1093/noajnl/vdz025
19. Han W, Yu F, Wang R, Guan W, Zhi F. Valproic Acid Sensitizes Glioma Cells to Luteolin Through Induction of Apoptosis and Autophagy via Akt Signaling. Cell Mol Neurobiol. 2021 Nov;41(8):1625-1634. https://doi.org/10.1007/s10571-020-00930-2
20. van der Meer PB, Koekkoek JAF. Valproic acid in glioma: Will the anticancer issue ever be solved? Neurooncol Pract. 2022 Nov 5;10(1):1-2. https://doi.org/10.1093/nop/npac091
21. Xuesong D, Wei X, Heng L, Xiao C, Shunan W, Yu G, Weiguo Z. Evaluation of neovascularization patterns in an orthotopic rat glioma model with dynamic contrast-enhanced MRI. Acta Radiol. 2017 Sep;58(9):1138-1146. https://doi.org/10.1177/0284185116681038
22. Toussi M, Isabelle B, Tcherny-Lessenot S, de Voogd H, Dimos V, Kaplan S. Effectiveness of risk minimisation measures for valproate: A cross-sectional survey among physicians in Europe. Pharmacoepidemiol Drug Saf. 2021 Mar;30(3):283-291. https://doi.org/10.1002/pds.5119
23. Schürmeyer L, Peng C, Albrecht W, Brecklinghaus T, Baur P, Hengstler JG, Schorning K. Design of optimal concentrations for in vitro cytotoxicity experiments. Arch Toxicol. 2025 Jan;99(1):357-376. https://doi.org/10.1007/s00204-024-03893-1
24. Jiwanti PK, Wardhana BY, Sutanto LG, Dewi DMM, Putri IZD, Savitri INI. Recent Development of Nano-Carbon Material in Pharmaceutical Application: A Review. Molecules. 2022 Nov 4;27(21):7578. https://doi.org/10.3390/molecules27217578
25. Krauze AV, Zhao Y, Li MC, Shih J, Jiang W, Tasci E, Cooley Zgela T, Sproull M, Mackey M, Shankavaram U, Tofilon P, Camphausen K. Revisiting Concurrent Radiation Therapy, Temozolomide, and the Histone Deacetylase Inhibitor Valproic Acid for Patients with Glioblastoma-Proteomic Alteration and Comparison Analysis with the Standard-of-Care Chemoirradiation. Biomolecules. 2023 Oct 10;13(10):1499. https://doi.org/10.3390/biom13101499
26. Woolley B, Mills J. Versatile Valproic Acid. Issues Ment Health Nurs. 2022 Nov;43(11):1072-1074. https://doi.org/10.1080/01612840.2022.2122431
27. Zhou C, Hu S, Botchway BOA, Zhang Y, Liu X. Valproic Acid: A Potential Therapeutic for Spinal Cord Injury. Cell Mol Neurobiol. 2021 Oct;41(7):1441-1452. https://doi.org/10.1007/s10571-020-00929-9
28. Singh D, Gupta S, Verma I, Morsy MA, Nair AB, Ahmed AF. Hidden pharmacological activities of valproic acid: A new insight. Biomed Pharmacother. 2021 Oct;142:112021. https://doi.org/10.1016/j.biopha.2021.112021
29. Khare P, Edgecomb SX, Hamadani CM, Tanner EEL, S Manickam D. Lipid nanoparticle-mediated drug delivery to the brain. Adv Drug Deliv Rev. 2023 Jun;197:114861. https://doi.org/10.1016/j.addr.2023.114861
30. Dey M, Singh RK. Exposure of aluminium to C6 glioma cells modulates molecular and functional neurotoxic markers. J Biochem Mol Toxicol. 2022 Dec;36(12):e23210. https://doi.org/10.1002/jbt.23210
31. Chen X, Wong JY, Wong P, Radany EH. Low-dose valproic acid enhances radiosensitivity of prostate cancer through acetylated p53-dependent modulation of mitochondrial membrane potential and apoptosis. Mol Cancer Res. 2011 Apr;9(4):448-61. https://doi.org/10.1158/1541-7786.MCR-10-0471
32. Xia Q, Zheng Y, Jiang W, Huang Z, Wang M, Rodriguez R, Jin X. Valproic acid induces autophagy by suppressing the Akt/mTOR pathway in human prostate cancer cells. Oncol Lett. 2016 Sep;12(3):1826-1832. https://doi.org/10.3892/ol.2016.4880
33. Michaelis M, Michaelis UR, Fleming I, Suhan T, Cinatl J, Blaheta RA, Hoffmann K, Kotchetkov R, Busse R, Nau H, Cinatl J Jr. Valproic acid inhibits angiogenesis in vitro and in vivo. Mol Pharmacol. 2004 Mar;65(3):520-7. https://doi.org/10.1124/mol.65.3.520