Original article
Ukrainian Neurosurgical Journal. 2024;30(4):30-42
https://doi.org/10.25305/unj.310430
Department of Neurosurgery, Bogomolets National Medical University, Kyiv, Ukraine
Received: 29 August 2024
Accepted: 27 September 2024
Address for correspondence:
Ziia K. Melikov, Department of Neurosurgery, Bogomolets National Medical University, 32 Platona Mayborody st., Kyiv, 04050, Ukraine, e-mail: melikov_ziya@ukr.net
Introduction. Peripheral nerve injury (PNI) is a common wartime pathology, the presence of which significantly complicates the course and treatment of combat injuries to the limbs. The development of new methods of treatment of PNI is impossible without validating existing models of PNI and clarifying the dynamics of the recovery process in this type of injury over long periods of observation. In this paper, the dynamics of the sciatic functional index (SFI) after transection and immediate suturing of the sciatic nerve of an adult rat during 24 weeks of observation was analyzed in detail.
Objective: to analyze the dynamics of SFI after transection, as well as after transection and immediate suturing of the sciatic nerve of an adult rat for 24 weeks and compare the obtained results with the data of other authors under similar experimental conditions.
Materials and Methods. The study was performed on 76 white adult outbred male rats, adhering to bioethical norms. In animals of the Sham group (n=24) an access to the sciatic nerve was performed, in animals of the Sect group (n=29) — the sciatic nerve was transected, and Raph group (n=23) — transection and immediate epineural suturing of the sciatic nerve was performed. A certain number of animals were removed from each group 4, 8, and 12 weeks after surgery for electrophysiological and morphological studies, and for the rest of the animals, the experiment was completed 24 weeks after the start of observation. SFI was determined before animals were removed, for all animals in each group at 4, 8, 12, 16, 20 and 24 weeks according to the Bain-Mackinnon-Hunter formula. Processing of digital data was carried out by various means of mathematical statistics.
Results. In animals of the Sham group, which were observed throughout the entire 24 weeks of the experiment (n=7), the average value of SFI one month after the injury simulation was -8.9 points and did not change significantly until the end of the experiment. In animals of the Sect group, which were observed throughout the entire 24 weeks of the experiment (n=8), one month after the injury, the mean SFI value was –84.7 points, significantly increasing to –67.0 points at the end of the 16th week, and subsequently significantly decreasing to –96.5 points. In animals of the Raph group, which were observed throughout the entire 24 weeks of the experiment (n=7), the average value of SFI after one month was -64.4 points, and its increase to -45.4 points at the end of week 24 should be considered relatively reliable. Pairwise comparison of the averaged for all animals SFI values in the Sham and Sect, Sham and Raph, and Sect and Raph groups revealed significant differences at 4, 8, 12, 20, and 24 weeks after simulated injury. At 16 weeks post-intervention, the SFI values in the Sect and Raph groups were significantly different from those in the Sham group, but were not different from each other.
Conclusions. The method of determining the function of the paretic limb after sciatic nerve injury in rats using SFI has a number of technical limitations, which are the reason for significant variability in experimental results among different research groups. The reliable biphasic SFI dynamics that was discovered after sciatic nerve transection, as well as the insignificant (according to this data) fluctuations in SFI after sciatic nerve transection and neurorrhaphy, require independent verification, pathophysiological interpretation, and should be taken into account when evaluating rehabilitation methods using such an experimental model of peripheral nerve injury.
Keywords: peripheral nerve injury; sciatic nerve transection, neurorrhaphy, sciatic nerve functional index, temporal dynamics of the indicator
Introduction
Peripheral nerve injury (PNI) is a common injury that often results in disability. Its prevalence, according to various sources, is approximately 3% of all injuries during peacetime [1-9] and around 5% when accounting for specific cases of plexus and spinal root injuries [8]. This translates to an incidence of 1–2 cases per 10,000 people annually [10-13], with even higher rates in developing countries [10]. During wartime, PNI frequently occurs as part of blast and gunshot injuries to the limbs, often accompanied by vascular and bone damage ([14–17] for peacetime gunshot PNIs, [18] - for peacetime PNIs in general, [19] - for wartime PNIs) significantly complicating the clinical course of this type of trauma.
PNI is generally considered the mildest form of nervous system injury but is characterized by a combination of prolonged sensory, motor, trophic, and pain disorders [1-8]. It also incurs substantial direct and indirect financial costs [4-7, 11, 20-26], which continue to rise annually [27]. Some studies have observed a decline in the proportion of PNI among peacetime injuries over the past 30 years [9]. Observational studies of patients with peripheral neuropathy indicate an increased risk of premature death [28]. However, it is unclear whether this is true for patients with post-traumatic neuropathy or if a direct link exists between PNI and mortality.
This type of trauma demonstrates age (average age 36–39 years [9, 11, 12, 27]) and sex specificity, occurring twice [11-13], three times [11, 18, 27, 29], or even four times [9] more often in men. The most common injuries involve the nerves of the upper limbs, particularly the wrist and hand [11-13, 18, 25, 27]. Left-sided injuries are reportedly more frequent [18, 25]. Treatment for PNI is primarily surgical [9, 12, 13], often performed urgently and typically involves direct anatomical repair of the nerve through neurorrhaphy [13].
Despite the relatively high regenerative potential of the peripheral nervous system, the plasticity of its central counterpart, and significant advances in PNI treatment, clinical outcomes remain suboptimal. Improvement is possible through a comprehensive approach targeting the injury site, the central nervous system, and paretic muscles [30]. Developing such methods requires experimental studies and standardization of PNI models [31-35].
The model of complete transection of the sciatic nerve is considered one of the most convenient for testing implant-based treatment approaches [31]. However, despite its relative simplicity, the reproducibility of results with this model is unsatisfactory, necessitating its validation. Moreover, there is limited information in the available literature on this model’s behavior over periods exceeding 90 days [36-40]. Similarly, data on the statistical analysis of temporal changes in the functional-anatomical indicator, the sciatic functional index (SFI), following nerve transection or segment excision in rats, are scarce [41, 42].
Objective: To analyze the dynamics of SFI following transection and immediate suturing of the sciatic nerve in adult rat over a 24-week observation period and compare the results with data from other authors under similar experimental conditions.
Materials and Methods
Experimental animals and groups
The study was conducted on 76 white outbred rats aged 4–6 months, weighing 280–380 g, sourced from the vivarium of the Romodanov Institute of Neurosurgery of the National Academy of Medical Sciences of Ukraine. The animals were housed under natural light conditions, with standard temperature and humidity levels, and fed a balanced combined diet ad libitum. During the study, principles of bioethics and humane treatment of animals were followed in accordance with the EU Council Directive 86/609/EEC on the approximation of laws, regulations and administrative provisions of the Member States regarding the protection of animals used for experimental and other scientific purposes (1986), the European Convention for the Protection of Vertebrate Animals Used for Experimental and Other Scientific Purposes (1986), and the Law of Ukraine No. 3447-IV “On the Protection of Animals from Cruelty” (2006). Approval for the study was obtained from the Bioethics and Ethics Commission for Scientific Research of Bogomolets National Medical University (minutes No. 155, dated January 31, 2022) and the Bioethics Committee of the Romodanov Institute of Neurosurgery of the National Academy of Medical Sciences of Ukraine (minutes No. 39, dated May 18, 2022).
Three experimental groups were formed: 1) a group of sham-operated animals that underwent only surgical access to the sciatic nerve (Sham; n=24); 2) a group of modelling a complete section of the sciatic nerve in the middle third (Sect; n=29); 3) a group of modelling a complete transection of the sciatic nerve in the middle third and its immediate epineural neurorrhaphy (Raph; n=23). A certain number of animals were removed from each group 4, 8 and 12 weeks after the surgical intervention for electrophysiological and morphological studies (Table 1). The remaining animals completed the experiment 24 weeks after the start of the observation period.
Table 1. Composition of experimental groups and the time course of animal removal from the experiment
|
Term of animals withdrawal from the experiment; weeks after the surgical intervention |
The initial number of animals in each group (given in the title of each column) and the number of animals in the group withdrawed from the experiment at each of the indicated observation time points (given in the cells of the table) |
||
|
Sham (n=24) |
Sect (n=29) |
Raph (n=23) |
|
|
4 |
5 |
8 |
5 |
|
8 |
6 |
6 |
5 |
|
12 |
6 |
7 |
6 |
|
24 |
7 |
8 |
7 |
Surgical Procedures
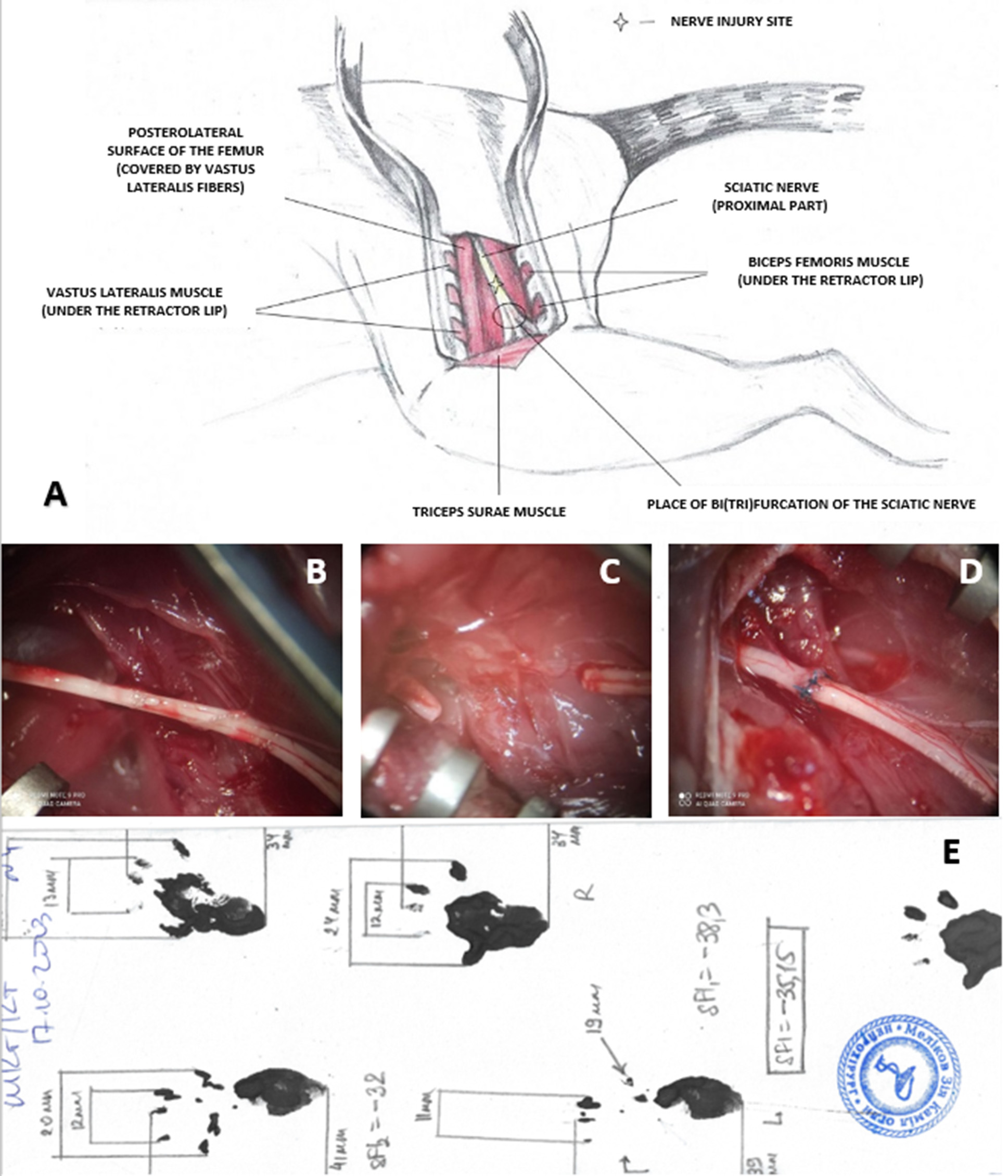
The surgical procedures were performed under general anesthesia, induced via intraperitoneal injection of a mixture of xylazine hydrochloride (15 mg/kg, «Bіowet», Poland) and ketamine hydrochloride (75 mg/kg, «Farmak», Ukraine). The adequacy of anesthesia was verified by the following criteria: absence of the corneal reflex, no withdrawal of the hind paw upon firm pressure on the foot, absence of whisker movements synchronized with the respiratory cycle, shallow rhythmic respiratory movements, and noticeable exophthalmos. Once these indicators were confirmed, the animal was positioned in a standard physiological posture (prone), with the limbs secured to the edges of the surgical table using cords. The skin on the posterolateral surface of the left thigh was shaved with scissors and treated with an antiseptic povidone-iodine solution («EGIS», Hungary). In moderately aseptic conditions, an incision was made along the line of the most superficial lateral surface of the femur. The attachment site of the tendon of the short head of the biceps femoris muscle was visualized, and a linear incision was made along the bone. The mobilized head of the muscle was retracted to the side (Figure 1, A). In the exposed pocket between the mobilized muscle head, bone, and other intact thigh muscles, the sciatic nerve trunk was identified and separated from surrounding tissues from the point where it exits the pelvic cavity to its bifurcation into main branches (Figure 1, B). In the Sham group, the surgical procedure ended at this stage by closing in layers of the dissected edge of the short head of the biceps femoris muscle and the tendinous portion of the vastus lateralis muscle at its attachment to the femur, followed by suturing of the skin edges with interrupted sutures (suture material No. 3-0, «Ethicon», USA). In the Sect group, the mobilized sciatic nerve trunk was transected using ophthalmic scissors, observing rapid retraction of the proximal stump (Figure 1, C). After ensuring hemostasis, the surgical procedure was completed as described above. In the Raph group, the stumps of the transected sciatic nerve were reconnected in an end-to-end manner using 3–6 (depending on nerve thickness) epineural interrupted sutures with moderate axial tension, performed with monofilament sutures (8.0–10.0, «Ethicon», USA) under 10–14x magnification of a surgical microscope (Figure 1, D). The procedure was completed as described above.

Fig. 1. Features of sciatic nerve injury modeling and SFI calculation in experimental animals:
A — Schematic representation of the surgical approach to the left sciatic nerve in a rat;
B–D — Intraoperative microphotographs of the surgical site after the main intervention stage:
B — Sciatic nerve trunk isolated from surrounding tissues (Sham group);
C — Sciatic nerve after transection, showing retraction and a gap between the proximal and distal
stumps (Sect group);
D — Sciatic nerve after transection and immediate reconnection of the stumps with five end-to-end
epineural interrupted sutures. Magnification ×14;
E — Example of SFI calculation using rat paw prints from the Raph group 12 weeks post-surgery.
SFI –35.15.
In all cases, the skin of the surgical wound was treated with povidone-iodine solution («Betadine®», «EGIS», Hungary). To prevent infectious complications, a solution of bicillin-5 («Arterium», Ukraine) was administered subcutaneously in the posterior cervical area at a dose of 1 million IU per 1 kg body weight. Anti-inflammatory and analgesic therapy included intraperitoneal administration of dexamethasone («KRKA», Slovenia) at a dose of 6 mg/kg body weight. After the described manipulations, the animals were kept in a room with an elevated air temperature for 2–4 hours until their behavioral activity resumed. Subsequently, they were housed under standard conditions in cages measuring 55 × 33 × 20 cm (length, width, height), with 3–6 animals per cage.
Determination of the sciatic functional index (SFI)
The SFI was measured in all experimental groups at 4, 8, 12, 16, 20, and 24 weeks post-operation. Testing was mandatory for animals at the specified time points before their removal from the experiment. The minimum testing period was set due to the unreliability of SFI measurements during the first 3 weeks following sciatic nerve injury in rats [43,44]. Differences between groups were identified for the 4th (4% of the observation period), 8th (11%), 12th (5%), and 24th (1%) weeks of observation. Footprints required for SFI calculation were obtained on a paper strip covering the floor of a tunneled horizontal runway [45–47]. Before testing, each animal was trained to move through the tunnel. After applying gouache to the plantar surface of the hind paws, the rat was released into the tunnel, which led to a cage. On the continuous, unidirectional gait footprints obtained, the distances between major anatomical points of the paw were measured, and SFI was calculated using the Bain–Mackinnon–Hunter formula [46]:
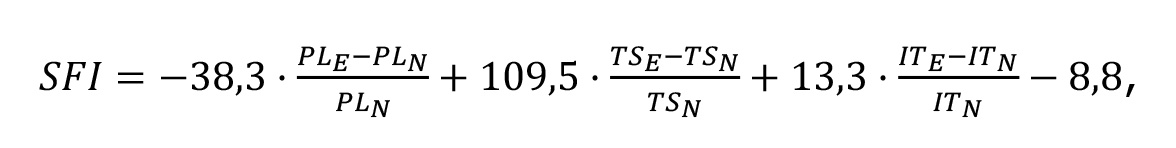
where E — injured limb; N — intact limb;
PL — distance from the heel to the longest toe;
TS — distance between the 1st and 5th toes;
IT — distance between the 2nd and 4th toes.
The SFI ranges from –100 points (reflecting footprints indicating a complete loss of sciatic nerve function) to 0 points (reflecting normal sciatic nerve function).
Exclusion Criteria
Animals showing signs of purulent-inflammatory complications, trophic ulcers on the paretic limb or adjacent areas, and/or signs of autophagy were removed from the experiment through chemical euthanasia (one animal from the Sect group on the 18th day post-operation).
In total, 16 animals died during the first 2 days after surgery due to unspecified reasons (6 animals from the Sham group, 6 from the Sect group, and 4 from the Raph group). Additionally, 3 animals died later (1 from the Sect group in the 5th month of observation and 2 from the Sect group during the 1st week of observation).
These animals, excluded from the experiment, were not included in the previously stated total number of experimental animals (76).
Statistical data analysis
Statistical analysis was performed using the EZR software package (R-Statistics), which is freely available online (https://www.softpedia.com/get/Science-CAD/EZR.shtml). The mean SFI values were presented as M±SD for samples with a normal distribution, where M (mean) represents the arithmetic mean, and SD (standard deviation) indicates the standard error of the mean. For samples without a normal distribution, data were presented as Me (QI-QIII), where Me (median) is the median, and QI–QIII are the first and third quartiles, respectively. The distribution type was determined using the Shapiro–Wilk test.
For animals observed over all 24 weeks, differences in SFI values at different observation time-points were analyzed using the Friedman test (for non-normally distributed SFI values) or repeated-measures ANOVA (rANOVA) with Bonferroni correction for multiple comparisons (for normally distributed SFI values). If in this way significant differences were detected within a group, additional pairwise comparisons between SFI values at different observation time-points were performed using the Student's t-test (for normally distributed data) or the Wilcoxon rank-sum test (for non-normal distributions).
To assess correlations between SFI values and observation time in animals, which were observed throughout the entire 24 weeks of the experiment, the Spearman rank correlation test was used for non-normally distributed SFI values (distribution of observation duration values was always non-normal), while Pearson’s test was applied for normally distributed SFI values. Bonferroni correction was applied for multiple comparisons.
To evaluate significant differences in SFI values between groups at specific time points, the normality of distribution was assessed using the Shapiro–Wilk test. If at least one sample deviated from a normal distribution, the Kruskal–Wallis test was used to assess group differences, followed by Steel–Dwass post hoc comparisons. For normally distributed SFI values, Bartlett's test was applied to compare variance homogeneity. If variances were non-normally distributed, the Kruskal–Wallis test and Steel–Dwass post hoc test were used. For normally distributed variances, ANOVA with Tukey’s post hoc test was applied to compare samples.
In all cases, a result was considered statistically significant if the probability of the null hypothesis was less than 0,05 (p < 0,05).
Results
The group-averaged values for animals observed throughout the 24 weeks of the experiment are presented in Table 2 and analyzed for significance of changes over time (Table 2, Fig. 2).
Table 2. Averaged SFI values in experimental groups obtained from the results of testing animals observed throughout all 24 weeks of the experiment
|
Observation period, week |
Experimental groups |
||
|
Sham (n = 7) |
Sect (n = 8) |
Raph (n = 7) |
|
|
Me (QI‒QIII) |
Me (QI‒QIII) |
Me (QI‒QIII) |
|
|
4 |
–8,93 |
–84,68 |
–64,4 |
|
8 |
–11,35 |
–80,98 |
–61,55 |
|
12 |
–4 |
–76,15 |
–63,38 |
|
16 |
–7,89 |
–66,95 |
–52,37 |
|
20 |
–11,2 |
–76,91 |
–56,3 |
|
24 |
–6,4 |
–96,48 |
–45,4 |
Note: *SFI values in the Sect group significantly differ from the values in this group at 16 weeks of observation; † The difference in SFI values in the Sect group between 16 and 24 weeks of observation is statistically significant.
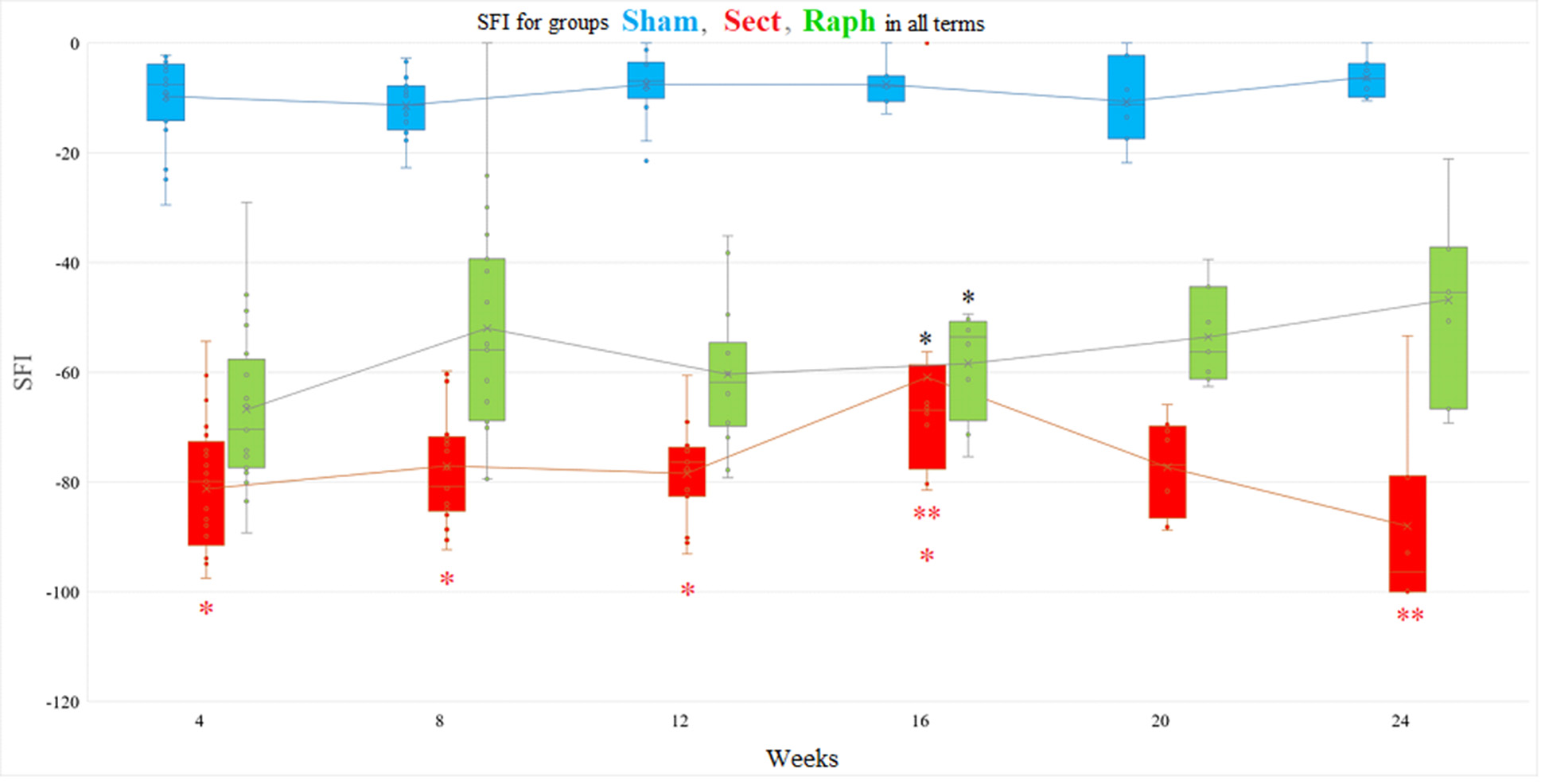
Fig. 2. Correlation between SFI values and the duration of observation in experimental groups (Sham — n=7, Sect — n=8, Raph — n=7; see Table 2). Upper row from left to right: Sham group — rS =0,28 (Spearman rank correlation test, p=0,07); Sect group — r=–0,06, 95% CI –0,34…+0,22 (Pearson’s test, p=0,67); Raph group — r=0,45, 95% CI +0,2... +0,7 (Pearson’s test, p<0,05). Lower row from left to right: Sect group, 4, 8, 12, and 16 weeks of observation — r=0,43, 95% CI +0,1… +0,7 (Pearson’s test, p=0,015); Sect group, 16, 20, and 24 weeks of observation — r=–0,58, 95% CI –0,8…–0,2 (Pearson’s test, p=0,003).
In the Sham group (n=7), the mean SFI value one month after injury modeling was –8,93 points (–16,7; –8,81) and did not significantly change throughout the experiment (p > 0.05, Friedman test with Bonferroni correction) (Table 2). This is supported by the absence of a correlation between SFI values and the duration of observation in this group (rS = 0,28, p > 0,05) (Fig. 2).
In the Sect group (n=8), the mean SFI value one month after injury modeling was –84,68 points (–93,67; –72,87). By the end of week 16, it increased to –66,95 points (–72,32; –64,52) (p < 0,05, Student’s t-test for pairwise comparisons with values at weeks 4, 8, and 12). However, it decreased again to –96,48 points (–100,0; –79,08) by the end of the experiment (p < 0,01, Wilcoxon T-test for pairwise comparisons between weeks 16 and 24) (Table 2). Thus, no significant correlation was found between SFI values and observation duration in this group (r = –0,06, 95% CI –0,34 to +0,22, p = 0,67). If two sub-periods, 4-16 and 16-24 weeks, are distinguished in the total follow-up period in the Sect group, a statistically significant average strength of association between SFI values and duration of follow-up was found for each of them (Fig. 2): positive correlation (r=0,43, 95% CI +0,1 to +0,7, p<0,05) and negative correlation (r=–0,58, 95% CI –0,8 to ‒0,2, p<0,01), respectively.
In the Raph group (n=7), the mean SFI value one month after injury modeling was –64,4 points (–77,65; –56,52), which non-significantly increased to –45,4 points (–58,7; –37,35) by the end of the experiment (Friedman test with Bonferroni correction, p > 0,05 for comparisons across observation periods) (Table 2). However, the presence of a moderate positive correlation between SFI values and observation duration (r = 0,45, 95% CI +0,2 to +0,7, p < 0,05) indicates the significance of this SFI dynamic (Fig. 2).
Pairwise intergoup comparisons of SFI values of all animals revealed statistically significant differences for all three group pairs (Sham and Sect, Sham and Raph, and Sect and Raph) at weeks 4, 8, 12, 20, and 24 after injury modeling (p < 0,05, Kruskal–Wallis test and Steel–Dwass post hoc comparisons) (Table 3; Fig. 3). At week 16 post-injury, SFI values in the Sect and Raph groups were significantly different from the Sham group (p < 0,05, ANOVA and Tukey’s post hoc test) but not from each other (p > 0,05, ANOVA and Tukey’s post hoc test) (Table 3; Fig. 3).
Table 3. Averaged SFI values of the experimental groups (obtained from the results of testing all animals) and their differences at each observation period
|
Observation period, week |
Experimental groups (the number of animals tested at each time point of the experiment is indicated in each corresponding cell of the table) |
||
|
Sham |
Sect |
Raph |
|
|
4 |
Me (QI–QIII), n=24 |
Me (QI–QIII), n=29 |
Me (QI–QIII), n=24 |
|
–7,6 (–13,91;–4,09) |
–79,9 (–89,85;–73,85) |
–70,41 (–77,42;–58,57) |
|
|
8 |
M±SD, n=19 |
M±SD, n=21 |
M±SD, n=19 |
|
–11,36±5,18 |
–77,15±10,1 |
–51,99±20,63 |
|
|
12 |
M±SD, n=13 |
M±SD, n=15 |
M±SD, n=14 |
|
–7,62±6,29 |
–78,49±8,64 |
–60,38±12,95 |
|
|
16 |
M±SD, n=7 |
M±SD, n=8 |
M±SD, n=8 |
|
–7,54±4,03 |
–68,58±8,67 ● |
–58,39±10,01 ● |
|
|
20 |
M±SD, n=7 |
M±SD, n=8 |
M±SD, n=7 |
|
–10,67±7,81 |
–77,34±8,82 |
–53,57±8,93 |
|
|
24 |
Me (QI–QIII), n=7 |
Me (QI–QIII), n=8 |
Me (QI–QIII), n=7 |
|
–6,4 (–9,08;–4,37) |
–96,48 (–100,0;–79,08) |
–45,4 (–66,7;–37,2) |
|
Note. ● The difference in SFI values between the Sect and Raph groups is statistically insignificant; the difference in SFI values when comparing all pairs at all other observation time points is statistically significant.
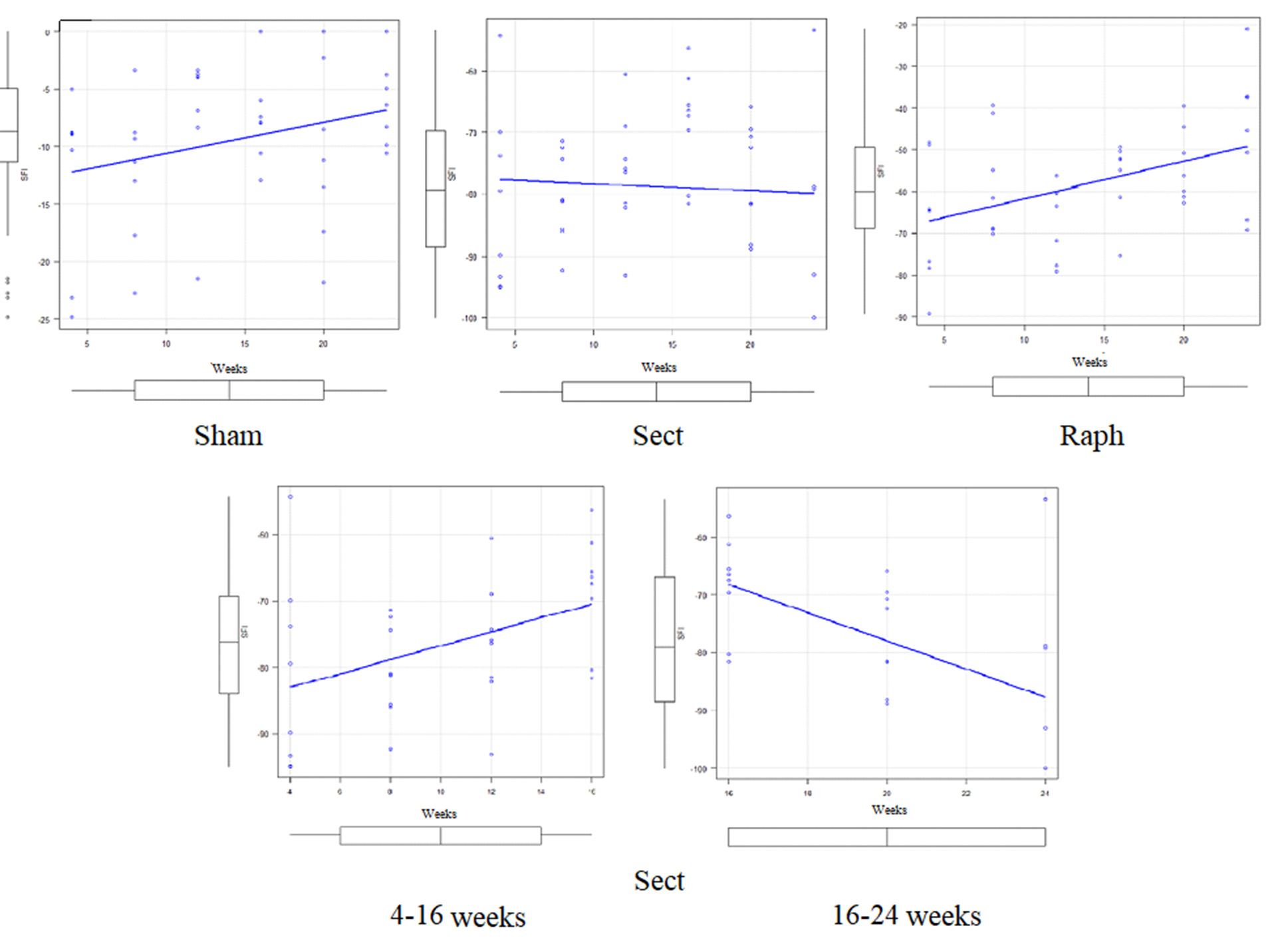
Fig. 3. Actual SFI values of all experimental animals (points; see Table 3), their medians (horizontal lines within the rectangles), the boundaries of the I and III quartiles (parts of the colored bar located below and above the median, respectively, at each time point), mean values (x), standard deviations (distance between the mean value marker and the lower or upper edge of the bar), and the degree of dispersion (variance) beyond the upper and lower quartiles (horizontal whisker bars) of the three experimental groups at all observation time points. The mean values at different observation time points for each group are connected by a solid line of the corresponding color, which only conditionally reflects the temporal dynamics, since in all groups at the same time points a certain number of rats were removed, reducing the number of animals in the group:
*(black, located on top) — the difference in SFI values between the Sect and Raph groups after 16 weeks of observation is statistically insignificant;
*(red, located below) — the difference in SFI values in the Sect group at 4, 8, and 12 weeks of observation compared to the value at 16 weeks of observation is statistically significant;
**(red, located below) — the difference in SFI values in the Sect group at 16 and 24 weeks of observation is statistically significant.
Discussion
Limitations of the method for determining the state of the paretic limb using SFI
The study of the effectiveness of any new method for restoring the function of an injured nerve is carried out under experimental conditions using various models of this pathology. Probably, the most common model today is the injury of the sciatic nerve of an adult rat, specifically its complete transection [31‒35, 48]. However, a significant number of questions remain unresolved (standardization of the surgical component, means of verifying nerve regeneration, clinical translation of the obtained results) [44].
Despite the mixed type of the sciatic nerve and the important role of somatosensory signaling in locomotion [49], researchers' attention is focused on monitoring the correlates of the integral motor capability of the paretic limb against the background of sciatic nerve injury. The most common of these is SFI, which reflects the anatomical features of the paretic foot under the condition of its loading during the free unidirectional locomotion of the animal. From this perspective, the index is a functional-anatomical indicator. The method of calculating SFI was proposed and algorithmized by L. De Medinaceli et al. (1982, 1984) [41, 50], subsequently modified and tested [46, 51]. Today, it can be stated that some studies indicate the absence of correlation between SFI values and morphometric indicators of the sciatic nerve in some models of its injury (reviewed [46]), while others demonstrate such a correlation ([42, 52], reviewed [43]). Importantly, SFI values correlate [51] with a more complex and subjective [53, 54] indicator of motor activity of the rat's hind limb, proposed by D.M. Basso, M.S. Beattie, J.C. Bresnahan for assessing motor deficit in animals against the background of spinal cord injury [54, 55]. This indicator may prove to be more sensitive for detecting residual motor deficit after certain types of sciatic nerve injury [51, 56, 57].
Despite the widespread use of SFI, it is important to remember a number of technical limitations of this method for determining the state of the paretic limb [46, 56]: 1) obtaining quality prints is possible only at a moderate speed of the animal passing through the track, 2) the increase in the animal's mass during the experiment changes the characteristics of the prints and can affect the result of the SFI calculation, 3) the clarity of the prints can be significantly distorted due to deformation and positioning of the paretic foot caused by contractures, as well as due to the consequences of autophagic or automutilative disruption of the foot's anatomy. For example, autophagy of the phalanges of the denervated foot [58] usually occurs starting from the third week after injury, is accompanied by infection, regional edema, and dystrophic changes in the foot tissue, and is considered a manifestation of post-traumatic complex regional pain syndrome [58, 59]. Obviously, dystrophic changes in the denervated foot can distort its prints, and chronic pain can cause protective locomotion to limit mechanical irritation, which also affects the prints, including those of the intact foot due to its compensatory overloading [46].
Given the mentioned factors, the relevance of the methodology for assessing the function of the rat's sciatic nerve using SFI is probably satisfactory only after 3 weeks of observation [43]. The individual variability of SFI values, characteristic of any study mentioned here, remains unexplained; its probable causes, among other things, may be individual differences in the segmental sources of nerve fibers of the sciatic nerve [60], and therefore, the muscles innervated by this nerve, as well as the disregard for the high branching of the nerve into branches [61].
Another feature of the modern variant of SFI calculation is the impossibility of its accurate determination in intact animals. For this reason, in the Sham group, the recovery of the SFI value to zero points was not registered. One of the explanations is the peculiarity of the SFI calculation formula, according to which, with complete symmetry of the prints of the hind feet of an intact animal, all members of the formula turn to zero except for the last term, so SFI under such conditions should be –8.8 points. The motivation of Bain, Mackinnon, and Hunter regarding this feature is not clarified due to the inaccessibility of their publication. However, according to E.F. Oliveira et al. (2001) [52], the fact that SFI in healthy intact animals with such a calculation does not equal zero may indicate the limited ability of this method to determine the state of the paretic limb.
Long-term monitoring of consequences is a key requirement from the perspective of quality and safety control of any new treatment method. Paradoxically, there is an extremely limited number of works in which, on the model of sciatic nerve transection and its immediate reconstruction, the state of the neuromuscular apparatus was assessed for more than 3 months, i.e., more than 12-13 weeks or 90 days ([36] — 14 weeks, [37] — 32 weeks, [40] — 24 weeks, [38] — 52 weeks, [39] — 5 months). In some of these works [37], there is no assessment of the motor activity of the paretic limb, at least using SFI.
Comparison of obtained data with results from other research groups
Given the mentioned reservations, it is necessary to compare the data we obtained with the results from other groups. A distinctive feature of the SFI value dynamics we observed is its multiphase nature: in the Sect group, the maximum value occurs at the 16th week of observation, while in the Raph group, it occurs at the 8th and 24th weeks. The phasic nature of SFI dynamics in similar experimental models has been noted in many available studies. For example, in the case of modeling the transection and immediate suturing of the sciatic nerve in rats, L. De Medinaceli et al. (1982) [41] found an increase in the SFI index (calculated using their primary formula) from less than –100 points to approximately –90 points on the 11th day of observation, followed by a decrease to approximately –100 points on the 32nd day. It is not known whether these temporal changes in SFI were statistically significant.
A similar dynamic with a peak on the 10th day of observation (approximately –75 points) was observed by A. Ganguly et al. (2017) [62] after complete transection of the sciatic nerve without neurosuturing in adult male Long Evans rats. In other strains under similar conditions and instruments, they recorded a decrease and stabilization of SFI values (from approximately –75 to –100 points, Wistar strain), a consistently low value (approximately –100 points, Sprague Dawley strain), a decrease by the 14th day and a gradual increase (from approximately –75 to approximately –100 points, Lewis and Fischer strains). The total observation period in this study was only 35 days. The authors did not perform any statistical verification of the described features of SFI dynamics. Notably, according to the same data, with digital registration and analysis of prints, SFI values were higher than with conventional (analog) methods.
In adult male Sprague Dawley rats, Y. Jung and colleagues (2014) [63], by analysing hind feet prints obtained by the analogue method, recorded an increase in the average SFI value from approximately –100 to about -75 points during the first week after a simulation of complete transection and immediate sciatic nerve suturing similar to ours, a decrease during the 2nd week and a gradual increase to approximately -60 points at the 12th week of the experiment.
According to J.K. Terzis and K.J. Smith (1987) [64], after transection and immediate suturing of the sciatic nerve in adult male Sprague Dawley rats, a biphasic dynamics of SFI (calculated using the primary method [41]) was recorded: from approximately –90 points in the 1st week to approximately –70 points in the 7th week and approximately –90 points in the 12th week of observation. The authors did not perform statistical verification of this dynamics. However, P.J. Evans et al. (1991) [36] under similar experimental conditions noted an increase in the SFI value to approximately –80 points by the end of the first month, a decrease over the next 2 weeks, a repeated increase to approximately –65 points by the end of the 10th week, a decrease by the end of the 12th week, and an increase to –40 points by the 14th week of observation. It is not known whether these changes were statistically significant.
After modeling complete transection and immediate suturing of the sciatic nerve in adult male Sprague Dawley rats, M. Sakuma et al. (2016) [65] detected two peaks in SFI values using digital print registration tools: a narrow peak at the beginning of the second month (approximately 6 weeks after injury modeling, approximately –100 points with an initial value of approximately –130 points) and around the 70th day (10 weeks after injury modeling, approximately –100 points with a value on the 90th day of approximately –120 points). Thus, under these experimental conditions, the authors did not find any signs of SFI recovery over a 3-month observation period. Strangely, the registration and analysis of prints using the same instrument after modeling complete transection of the sciatic nerve even without neurorrhaphy, according to A. Ganguly et al. (2017) [62] for the Sprague Dawley strain, yielded a much higher SFI value on the 35th day—approximately –40 points compared to approximately –120 points [65].
Therefore, after complete transection and immediate suturing of the sciatic nerve in adult male rats, some authors did not find signs of SFI recovery at the beginning of the second month [41] or up to the 12th [64] or 13th week [65], while others recorded it only after 12 weeks [63] or 14 weeks [36]. We were unable to find results from longer studies of SFI levels under these experimental conditions.
Significant discrepancies in SFI monitoring results are also characteristic of: 1) the model of sciatic nerve transection without neurorrhaphy performed on different rat strains [62], 2) obtaining SFI by conventional and digital methods [62], 3) obtaining prints and calculating SFI using the same system, but after sciatic nerve transection without neurorrhaphy (better results — [62]) and with immediate neurorrhaphy (worse results — [65]).
In the study by V.Y. Molotkovets et al. (2020) [39], following isolated transection of the sciatic nerve in adult male rats, the SFI value changed from –(79,3±3,8) points after 1 month post-injury to –(75,0±2,9) points at the end of the 3rd month, and to –(73,2±5,4) points at the end of the 5th month. In the case of immediate neurorrhaphy (we provide corrected data due to the presence of an editorial error [39]), the values changed from –(41,6±3,7) points at the end of the first month to –(33,2±4,4) points at the end of the 3rd month, and to –(21,3±1,2) points at the end of the 5th month of observation, which significantly differs from our data and the data of other authors.
Under similar experimental conditions, O. Goncharuk et al. (2020) [66] found that during the first month after isolated transection of the sciatic nerve in adult male rats, the SFI values were approximately –70 points. In the case of immediate suturing, the values gradually increased, almost linearly, from approximately –70 points 1 week post-injury to approximately –35 points after 4 weeks of observation.
Therefore, our data on SFI recovery following transection and immediate suturing of the rat's sciatic nerve (approximately –60 points at the 12th week) are consistent with the data of Y. Jung et al. (2014) [63] and P.J. Evans et al. (1991) [36], while the results of O. Goncharuk et al. (2020) [66] and V.Y. Molotkovets et al. (2020) [39] indicate much better SFI recovery outcomes.
Heterogeneous dynamics of SFI values can also be observed in the case of autoplasty of the transected sciatic nerve. For example, in a model of excising an 8-millimeter segment of the sciatic nerve and immediately closing the resulting defect with the same fragment in Fischer rats, T. Meder et al. (2021) [40] describe a biphasic dynamics of SFI—an increase from approximately –80 points at the 2nd week of observation to approximately –50 points at the 7th week, a slight decrease by the 10th week, a stable value until the 20th week, and an increase to approximately –50 points by the 24th week of observation. The authors did not determine the statistical significance of all these changes.
The statistical significance of SFI dynamics after sciatic nerve injury remains unexplored. Only one study contains an analysis of the reliability of temporal changes in SFI [42], but in this study, the observation of the injury lasted only 6 weeks. A crush injury, rather than transection of the rat's sciatic nerve, was reproduced. This issue is tangentially addressed in the model of sciatic nerve injury and immediate suturing in rats by L. De Medinaceli et al. (1982) [41], as well as by J.M. Shenaq et al. (1989) in a model of excision and immediate repair of a one-centimeter defect of the rat's sciatic nerve [67]. Attempts to analyze the statistical significance of differences in SFI values at three observation time points (1, 3, and 5 months) after transection or transection and immediate suturing of the rat's sciatic nerve were also made by V.Y. Molotkovets et al. (2020) [39], but the results of this analysis are not provided in the cited publication. Moreover, the limited number of time points does not reveal all the features of the recovery process dynamics. However, the authors found a statistically significant difference between SFI values at the end of the 3rd and 5th months, but not between the values at the end of the 1st and 3rd months. This observation is consistent with the data of other authors [36] and, to some extent, with our data, which indicate that in the case of transection and immediate suturing of the adult rat's sciatic nerve, a large increase in SFI values (irreversible until the end of observation in each of these experiments) occurs in the late period of the injury, no earlier than the 4th month.
Pathophysiological assumptions and speculative interpretation of the obtained data
It is known that regenerative growth of nerve fibers through the injury zone begins as early as the fourth day after transection and immediate suturing of the sciatic nerve in adult male Sprague Dawley rats. This growth occurs at a rate of 3,2 mm/day, as determined for sensory fibers by studying mechanical sensitivity along the exposed nerve trunk ("pinch test") [68]. Regenerative growth of motor fibers into the portion of the sciatic nerve distal to the crush zone in animals of a similar strain, sex, and age was observed starting from the third day. This was identified through the accumulation of radioactive-labeled (³H) proline in growth cones, previously stereotactically introduced into the anterior horn of the corresponding spinal cord segment. The growth rate of motor fibers ranged from 3,0 to 4,4 mm/day [69]. In mice, the regeneration of large fibers (motor and sensory) starts later and is generally less effective than the regeneration of small, autonomic, and pain fibers [70]. According to these data, knowing the distance from the injury site to the innervation zone, it can be stated that the first fibers will reach the innervation zone of the corresponding muscles after injury and immediate neurorrhaphy of the sciatic nerve in adult rats between the third or fourth day and the end of the first month.
Overall, in our opinion, interpreting the dynamics of the sciatic functional index (SFI) during the first month after sciatic nerve injury should consider the following factors: the rate of regenerative nerve fiber growth, sprouting reinnervation of paretic muscles by fibers from the intact femoral nerve, plasticity at various levels of the motor system, the potential development of pain syndrome, functional compensation of weight-bearing by the intact hind limb, immunogenic demyelination, and remyelination of nerve fibers (reviewed in [30, 44]), alongside the low reliability of the SFI during the first three weeks of observation. For instance, the peak SFI values recorded in some studies [41, 62, 63] during the second week of observation may indicate temporary compensation due to either preserved innervation of the paretic limb muscles for various reasons or additional loading of the intact limb. The rapid decline in SFI may be related to the exhaustion of this compensatory mechanism.
During the second month, the active establishment of functionally significant contacts between regenerating nerve fibers and muscles likely continues (see [37]), which is assumed to influence changes in the SFI. There is evidence that the regeneration of small autonomic and pain fibers begins earlier and yields greater functional outcomes (regarding their respective functions) than the regeneration of large-caliber fibers (motor and sensory) [69]. At this stage and beyond, plasticity processes in both central and peripheral parts of the motor system, including muscles and neuromuscular synapses, play a significant role (reviewed in [30, 44]). Additionally, the dynamics of the trauma zone's organization process may be relevant. Scar consolidation could theoretically contribute to demyelination, reduced conduction velocity, complete or partial action potential blockage, or even the death of individual nerve fibers. The exhaustion of motor neurons overloaded by primary compensatory activity redistribution, which maintained or initially restored connections with the paretic limb muscles, is also possible. If such motor neurons die, it will result in a decrease in the SFI, as observed in the Raph group during the third month. In the Sect group, similar dynamics were observed later, suggesting that processes characteristic of the Raph group in the second and third months may occur in the Sect group during the fourth and fifth months.
The near-linear increase in SFI after sciatic nerve transection and immediate suturing ([39]—over the first five months, [66]—over the first month) or for certain rat strains after sciatic nerve crush modeling ([62]—during the second and third weeks) could be explained by a linear increase in the number of motor fibers reinnervating damaged muscles during the specified period. This would involve a "wedge-shaped" growth front of these fiber groups at a uniform speed but with varying onset times for individual fiber growth. This scenario would resemble one of the mechanisms of ontogenetic growth in nerve fiber bundles, with leaders and followers among them [71, 72]. However, it is doubtful that the sensitivity of the SFI allows for such fine detection of changes in muscle innervation volume.
In general, it can be stated that identifying the role of each of these pathophysiological components in SFI dynamics is currently impossible due to limited knowledge about the molecular and cellular mechanisms of nerve regeneration and the inability to simultaneously monitor each component in real-time. Therefore, the most promising research designs should include not only SFI monitoring but also molecular-genetic, electrophysiological, morphological, and other modern methods applied concurrently.
Conclusions
Despite the relative simplicity of modeling, PNI remains poorly studied. One of the most common models of PNI is the transection of the sciatic nerve in adult rats. The most widely used and currently irreplaceable method for assessing the function of the paretic limb under these experimental conditions is the calculation of the SFI. However, literature data on the behavior of this parameter in this type of PNI vary significantly, are mostly limited to the first three months of observation, and often fail to reveal the reliability of SFI dynamics. According to our findings, in the case of sciatic nerve transection in adult rats, SFI values change in a biphasic manner, with a statistically significant peak at the 16th week of observation. In contrast, in the case of transection followed by immediate suturing, the SFI dynamics differ significantly, showing two statistically insignificant peaks at the 8th and 24th weeks of the experiment.
A comparison of our data with the results of other groups highlights the limitations of the methodology for studying sciatic nerve injury using SFI. We believe that improving this methodology should involve not only the development of more precise methods of lifetime monitoring of paretic limb function—combined with molecular-genetic, electrophysiological, and morphological analysis—but also the application of relevant statistical methods to assess the significance of temporal changes in the identified parameters over extended observation periods.
Disclosure
Conflict of Interest
The authors declare no conflicts of interest.
Ethical Standards
All procedures performed on experimental animals during the study complied with ethical standards and were approved by the ethics committee of the scientific institution where the research was conducted.
Funding
The study received no external sponsorship.
References
1. Kouyoumdjian JA. Peripheral nerve injuries: a retrospective survey of 456 cases. Muscle Nerve. 2006 Dec;34(6):785-8. https://doi.org/10.1002/mus.20624
2. Taylor CA, Braza D, Rice JB, Dillingham T. The incidence of peripheral nerve injury in extremity trauma. Am J Phys Med Rehabil. 2008 May;87(5):381-5. https://doi.org/10.1097/ PHM.0b013e31815e6370
3. Scholz T, Krichevsky A, Sumarto A, Jaffurs D, Wirth GA, Paydar K, Evans GR. Peripheral nerve injuries: an international survey of current treatments and future perspectives. J Reconstr Microsurg. 2009 Jul;25(6):339-44. https://doi.org/10.1055/s- 0029-1215529
4. Antoniadis G, Kretschmer T, Pedro MT, König RW, Heinen CP, Richter HP. Iatrogenic nerve injuries: prevalence, diagnosis and treatment. Dtsch Arztebl Int. 2014 Apr 18;111(16):273- 9. https://doi.org/10.3238/arztebl.2014.0273
5. Castillo-Galván ML, Martínez-Ruiz FM, de la Garza-Castro O, Elizondo-Omaña RE, Guzmán-López S. [Study of peripheral nerve injury in trauma patients]. Gac Med Mex. 2014 NovDec;150(6):527-32. Spanish
6. Missios S, Bekelis K, Spinner RJ. Traumatic peripheral nerve injuries in children: epidemiology and socioeconomics. J Neurosurg Pediatr. 2014 Dec;14(6):688-94. https://doi.org/10.3171/2014.8.PEDS14112
7. Bekelis K, Missios S, Spinner RJ. Falls and peripheral nerve injuries: an age-dependent relationship. J Neurosurg. 2015 Nov;123(5):1223-9. https://doi.org/10.3171/2014.11.JNS142111
8. Dalamagkas K, Tsintou M, Seifalian A. Advances in peripheral nervous system regenerative therapeutic strategies: A biomaterials approach. Mater Sci Eng C Mater Biol Appl. 2016 Aug 1;65:425-32. https://doi.org/10.1016/j.msec.2016.04.048
9. Zaidman M, Novak CB, Midha R, Dengler J. Epidemiology of peripheral nerve and brachial plexus injuries in a trauma population. Can J Surg. 2024 Jun 26;67(3):E261-E268. https://doi.org/10.1503/cjs.002424
10. Jiang L, Jones S, Jia X. Stem Cell Transplantation for Peripheral Nerve Regeneration: Current Options and Opportunities. Int J Mol Sci. 2017 Jan 5;18(1). pii: E94. https://doi.org/10.3390/ijms18010094
11. Tapp M, Wenzinger E, Tarabishy S, Ricci J, Herrera FA. The Epidemiology of Upper Extremity Nerve Injuries and Associated Cost in the US Emergency Departments. Ann Plast Surg. 2019 Dec;83(6):676-680. https://doi.org/10.1097/SAP.0000000000002083
12. Kim SJ, Kwon YM, Ahn SM, Lee JH, Lee CH. Epidemiology of upper extremity peripheral nerve injury in South Korea, 2008 to 2018. Medicine (Baltimore). 2022 Dec 2;101(48):e31655. https://doi.org/10.1097/MD.0000000000031655
13. Murphy RNA, de Schoulepnikoff C, Chen JHC, Columb MO, Bedford J, Wong JK, Reid AJ. The incidence and management of peripheral nerve injury in England (2005-2020). J Plast Reconstr Aesthet Surg. 2023 May;80:75-85. https://doi.org/10.1016/j.bjps.2023.02.017
14. Omid R, Stone MA, Zalavras CG, Marecek GS. Gunshot Wounds to the Upper Extremity. J Am Acad Orthop Surg. 2019 Apr 1;27(7):e301-e310. https://doi.org/10.5435/JAAOS-D-17-00676
15. Baker HP, Straszewski AJ, Dahm JS, Dickherber JL, Krishnan P, Dillman DB, Strelzow JA. Gunshot-related lower extremity nerve injuries. Eur J Orthop Surg Traumatol. 2023 May;33(4):851-856. https://doi.org/10.1007/s00590-022-03220-3
16. Dugom PM, Jester MP, Archie WH, Huynh DM, Scarcella JF, Guo Y. Outcomes in Ballistic Injuries to the Hand: Fractures and Nerve/Tendon Damage as Predictors of Poor Outcomes. Hand (N Y). 2024 May;19(3):382-386. https://doi.org/10.1177/15589447221092111
17. Muss TE, Hu S, Bauder AR, Lin IC. The Epidemiology, Management, and Outcomes of Civilian Gunshot Wounds to the Upper Extremity at an Urban Trauma Center. Plast Reconstr Surg Glob Open. 2024 Apr 17;12(4):e5753. https://doi.org/10.1097/GOX.0000000000005753
18. Aman M, Zimmermann KS, Thielen M, Thomas B, Daeschler S, Boecker AH, Stolle A, Bigdeli AK, Kneser U, Harhaus L. An Epidemiological and Etiological Analysis of 5026 Peripheral Nerve Lesions from a European Level I Trauma Center. J Pers Med. 2022 Oct 8;12(10):1673. https://doi.org/10.3390/jpm12101673
19. Shaprynskyi Y, Lypkan V. Treatment of patients with gunshot traumatic amputations of the lower limbs due to explosive injury in the conditions of today’s war in Ukraine. Reports of Vinnytsia National Medical University. 2023;27:581-585. 10.31393/reports-vnmedical-2023-27(4)-08
20. Rosberg HE, Carlsson KS, Höjgård S, Lindgren B, Lundborg G, Dahlin LB. Injury to the human median and ulnar nerves in the forearm--analysis of costs for treatment and rehabilitation of 69 patients in southern Sweden. J Hand Surg Br. 2005 Feb;30(1):35-9. https://doi.org/10.1016/j.jhsb.2004.09.003
21. Immerman I, Price AE, Alfonso I, Grossman JA. Lower extremity nerve trauma. Bull Hosp Jt Dis (2013). 2014;72(1):43-52.
22. Wali AR, Park CC, Brown JM, Mandeville R. Analyzing costeffectiveness of ulnar and median nerve transfers to regain forearm flexion. Neurosurg Focus. 2017 Mar;42(3):E11. https://doi.org/10.3171/2016.12.FOCUS16469
23. Foster CH, Karsy M, Jensen MR, Guan J, Eli I, Mahan MA. Trends and Cost-Analysis of Lower Extremity Nerve Injury Using the National Inpatient Sample. Neurosurgery. 2019 Aug 1;85(2):250-256. https://doi.org/10.1093/neuros/nyy265
24. Khalifeh JM, Dibble CF, Dy CJ, Ray WZ. Cost-Effectiveness Analysis of Combined Dual Motor Nerve Transfers versus Alternative Surgical and Nonsurgical Management Strategies to Restore Shoulder Function Following Upper Brachial Plexus Injury. Neurosurgery. 2019 Feb 1;84(2):362-377. https://doi.org/10.1093/neuros/nyy015
25. Bergmeister KD, Große-Hartlage L, Daeschler SC, Rhodius P, Böcker A, Beyersdorff M, Kern AO, Kneser U, Harhaus L. Acute and long-term costs of 268 peripheral nerve injuries in the upper extremity. PLoS One. 2020 Apr 6;15(4):e0229530. https://doi.org/10.1371/journal.pone.0229530
26. Raizman NM, Endress RD, Styron JF, Emont SL, Cao Z, Park LI, Greenberg JA. Procedure Costs of Peripheral Nerve Graft Reconstruction. Plast Reconstr Surg Glob Open. 2023 Apr 10;11(4):e4908. https://doi.org/10.1097/GOX.0000000000004908
27. Karsy M, Watkins R, Jensen MR, Guan J, Brock AA, Mahan MA. Trends and Cost Analysis of Upper Extremity Nerve Injury Using the National (Nationwide) Inpatient Sample. World Neurosurg. 2019 Mar;123:e488-e500. https://doi.org/10.1016/j.wneu.2018.11.192
28. Hicks CW, Wang D, Matsushita K, Windham BG, Selvin E. Peripheral Neuropathy and All-Cause and Cardiovascular Mortality in U.S. Adults : A Prospective Cohort Study. Ann Intern Med. 2021 Feb;174(2):167-174. https://doi.org/10.7326/M20-1340
29. Trehan SK, Model Z, Lee SK. Nerve Repair and Nerve Grafting. Hand Clin. 2016 May;32(2):119-25. https://doi.org/10.1016/j.hcl.2015.12.002
30. Melikov ZK, Medvediev VV. Peripheral nerve injury: molecular pathophysiology and prospects for restorative treatment by means of cell transplantation: a literature review. Ukr Neurosurg J. 2023Dec.26;29(4):3-12. https://doi.org/10.25305/unj.288785
31. Geuna S. The sciatic nerve injury model in pre-clinical research. J Neurosci Methods. 2015 Mar 30;243:39-46. https://doi.org/10.1016/j.jneumeth.2015.01.021
32. Gordon T, Borschel GH. The use of the rat as a model for studying peripheral nerve regeneration and sprouting after complete and partial nerve injuries. Exp Neurol. 2017 Jan;287(Pt 3):331-347. https://doi.org/10.1016/j.expneurol.2016.01.014
33. Vela FJ, Martínez-Chacón G, Ballestín A, Campos JL, Sánchez-Margallo FM, Abellán E. Animal models used to study direct peripheral nerve repair: a systematic review. Neural Regen Res. 2020 Mar;15(3):491-502. https://doi.org/10.4103/1673-5374.266068
34. Li A, Pereira C, Hill EE, Vukcevich O, Wang A. In Vitro, In Vivo and Ex Vivo Models for Peripheral Nerve Injury and Regeneration. Curr Neuropharmacol. 2022;20(2):344-361. https://doi.org/10.2174/1570159X19666210407155543
35. Varier P, Raju G, Madhusudanan P, Jerard C, Shankarappa SA. A brief review of in vitro models for injury and regeneration in the peripheral nervous system. International Journal of Molecular Sciences. 2022 Jan 13;23(2):816. https://doi.org/10.3390/ijms23020816
36. Evans PJ, Bain JR, Mackinnon SE, Makino AP, Hunter DA. Selective reinnervation: a comparison of recovery following microsuture and conduit nerve repair. Brain Res. 1991 Sep 20;559(2):315-21. https://doi.org/10.1016/0006-8993(91)90018-q
37. Meyer RS, Abrams RA, Botte MJ, Davey JP, Bodine-Fowler SC. Functional recovery following neurorrhaphy of the rat sciatic nerve by epineurial repair compared with tubulization. J Orthop Res. 1997 Sep;15(5):664-9. https://doi.org/10.1002/jor.1100150506
38. Meek MF, Den Dunnen WF, Schakenraad JM, Robinson PH. Long-term evaluation of functional nerve recovery after reconstruction with a thin-walled biodegradable poly (DL-lactide-epsilon-caprolactone) nerve guide, using walking track analysis and electrostimulation tests. Microsurgery. 1999;19(5):247-53. https://doi.org/10.1002/(sici)1098-2752(1999)19:5<247::aid-micr7>3.0.co;2-e
39. Molotkovets VY, Medvediev VV, Korsak AV, Chaikovsky YuB, Tsymbaliuk VI. Restoration of the Integrity of a Transected Peripheral Nerve with the Use of an Electric Welding Technology. Neurophysiology. 2020;52, 31–42. https://doi.org/10.1007/s11062-020-09848-3
40. Meder T, Prest T, Skillen C, Marchal L, Yupanqui VT, Soletti L, Gardner P, Cheetham J, Brown BN. Nerve-specific extracellular matrix hydrogel promotes functional regeneration following nerve gap injury. NPJ Regen Med. 2021 Oct 25;6(1):69. https://doi.org/10.1038/s41536-021-00174-8
41. de Medinaceli L, Freed WJ, Wyatt RJ. An index of the functional condition of rat sciatic nerve based on measurements made from walking tracks. Exp Neurol. 1982 Sep;77(3):634-43. https://doi.org/10.1016/0014-4886(82)90234-5
42. Wang T, Ito A, Aoyama T, Nakahara R, Nakahata A, Ji X, Zhang J, Kawai H, Kuroki H. Functional evaluation outcomes correlate with histomorphometric changes in the rat sciatic nerve crush injury model: A comparison between sciatic functional index and kinematic analysis. PLoS One. 2018 Dec 12;13(12):e0208985. https://doi.org/10.1371/journal.pone.0208985
43. Monte-Raso VV, Barbieri CH, Mazzer N, Yamasita AC, Barbieri G. Is the Sciatic Functional Index always reliable and reproducible? Journal of Neuroscience Methods. 2008;170(2):255-61. https://doi.org/10.1016/j.jneumeth.2008.01.022
44. Tsymbaliuk VI, Medvediev VV, Ivanchov PV, Molotkovets VY, Chaikovsky YB, Korsak AV. [Electrical welding technology in restoring the integrity of the injured peripheral nerve: review of literature and own experimental research]. Ukr Neurosurg J. 2020;26(2):24-33 Ukrainian. https://doi.org/10.25305/unj.199507
45. Dellon ES, Dellon AL. Functional assessment of neurologic impairment: track analysis in diabetic and compression neuropathies. Plast Reconstr Surg. 1991 Oct;88(4):686-94. PubMed PMID: 1896540
46. Varejão AS, Meek MF, Ferreira AJ, Patrício JA, Cabrita AM. Functional evaluation of peripheral nerve regeneration in the rat: walking track analysis. J Neurosci Methods. 2001 Jul 15;108(1):1-9. Review. PubMed PMID: 11459612
47. Tsymbalyuk VI, Petriv TI, Molotkovets VY, Medvedev VV, Luzan BM, inventors; Bogomolets National Medical University, assignee. The device for conducting the "walk on the track" test. Patent of Ukraine 118157. 2017 July 27.
48. Rigoni M, Montecucco C. Animal models for studying motor axon terminal paralysis and recovery. Journal of Neurochemistry. 2017;142:122-9. https://doi.org/10.1111/jnc.13956
49. Frigon A, Akay T, Prilutsky BI. Control of Mammalian Locomotion by Somatosensory Feedback. Compr Physiol. 2021 Dec 29;12(1):2877-2947. https://doi.org/10.1002/cphy.c210020
50. de Medinaceli L, DeRenzo E, Wyatt RJ. Rat sciatic functional index data management system with digitized input. Comput Biomed Res. 1984 Apr;17(2):185-92. https://doi.org/10.1016/0010-4809(84)90031-4
51. Schiaveto de Souza A, da Silva CA, Del Bel EA. Methodological evaluation to analyze functional recovery after sciatic nerve injury. J Neurotrauma. 2004 May;21(5):627-35. https://doi.org/10.1089/089771504774129955
52. Oliveira EF, Mazzer N, Barbieri CH, Selli M. Correlation between functional index and morphometry to evaluate recovery of the rat sciatic nerve following crush injury: experimental study. J Reconstr Microsurg. 2001 Jan;17(1):69-75. https://doi.org/10.1055/s-2001-12691
53. Abdallah I, Мedvediev V, Draguntsova N, Voitenko N, Tsymbaliuk V. Dependence of the restorative effect of Macroporous poly(N-[2-Hydroxypropyl]-methacrylamide hydrogel on the severity of experimental lacerative spinal cord injury. USMYJ. 2021 Dec. 26;127(4):8-21. https://doi.org/10.32345/USMYJ.127(4).2021.8-21
54. Medvediev VV, Abdallah IM, Draguntsova NG, Savosko SI, Vaslovych VV, Tsymbaliuk VI, Voitenko NV. Model of spinal cord lateral hemi-excision at the lower thoracic level for the tasks of reconstructive and experimental neurosurgery. Ukr Neurosurg J. 2021Sep.27;27(3):33-5. https://doi.org/10.25305/unj.234154
55. Basso DM, Beattie MS, Bresnahan JC. A sensitive and reliable locomotor rating scale for open field testing in rats. J Neurotrauma. 1995 Feb;12(1):1-21. https://doi.org/10.1089/neu.1995.12.1
56. Dinh P, Hazel A, Palispis W, Suryadevara S, Gupta R. Functional assessment after sciatic nerve injury in a rat model. Microsurgery. 2009;29(8):644-9. https://doi.org/10.1002/micr.20685
57. Amniattalab A, Mohammadi R. Functional, Histopathological and Immunohistichemical Assessments of Cyclosporine A on Sciatic Nerve Regeneration Using Allografts: A Rat Sciatic Nerve Model. Bull Emerg Trauma. 2017 Jul;5(3):152-159.
58. Wall PD, Devor M, Inbal R, Scadding JW, Schonfeld D, Seltzer Z, Tomkiewicz MM. Autotomy following peripheral nerve lesions: experimental anaesthesia dolorosa. Pain. 1979 Oct;7(2):103-11. https://doi.org/10.1016/0304-3959(79)90002-2
59. Coderre TJ, Grimes RW, Melzack R. Autotomy following sciatic and saphenous nerve sections - sparing of the medial toes after treatment of the sciatic-nerve with capsaicin. Experimental Neurology. 1986;91(2):355-65. HTTPS://DOI.ORG/10.1016/0014-4886(86)90075-0
60. Asato F, Butler M, Blomberg H, Gordh T. Variation in rat sciatic nerve anatomy: implications for a rat model of neuropathic pain. J Peripher Nerv Syst. 2000 Mar;5(1):19-21. https://doi.org/10.1046/j.1529-8027.2000.00155.x
61. Rupp A, Schmahl W, Lederer W, Matiasek K. Strain differences in the branching of the sciatic nerve in rats. Anat Histol Embryol. 2007 Jun;36(3):202-8. https://doi.org/10.1111/j.1439-0264.2007.00751.x
62. Ganguly A, McEwen C, Troy EL, Colburn RW, Caggiano AO, Schallert TJ, Parry TJ. Recovery of sensorimotor function following sciatic nerve injury across multiple rat strains. J Neurosci Methods. 2017 Jan 1;275:25-32. https://doi.org/10.1016/j.jneumeth.2016.10.018
63. Jung Y, Ng JH, Keating CP, Senthil-Kumar P, Zhao J, Randolph MA, Winograd JM, Evans CL. Comprehensive evaluation of peripheral nerve regeneration in the acute healing phase using tissue clearing and optical microscopy in a rodent model. PLoS One. 2014 Apr 8;9(4):e94054. https://doi.org/10.1371/journal.pone.0094054
64. Terzis JK, Smith KJ. Repair of severed peripheral nerves: comparison of the "de Medinaceli" and standard microsuture methods. Exp Neurol. 1987 Jun;96(3):672-80. https://doi.org/10.1016/0014-4886(87)90228-7
65. Sakuma M, Gorski G, Sheu SH, Lee S, Barrett LB, Singh B, Omura T, Latremoliere A, Woolf CJ. Lack of motor recovery after prolonged denervation of the neuromuscular junction is not due to regenerative failure. Eur J Neurosci. 2016 Feb;43(3):451-62. https://doi.org/10.1111/ejn.13059
66. Goncharuk O, Savosko S, Petriv T, Tatarchuk M, Medvediev V, Tsymbaliuk V. Epineurial sutures, polyethylene glycol hydrogel and fibrin glue in the sciatic nerve repair in rats: functional and morphological assessments in experiment. Georgian Med News. 2020 Dec;(309):124-131.
67. Shenaq JM, Shenaq SM, Spira M. Reliability of sciatic function index in assessing nerve regeneration across a 1 cm gap. Microsurgery. 1989;10(3):214-9. https://doi.org/10.1002/micr.1920100315
68. Forman DS, Wood DK, DeSilva S. Rate of regeneration of sensory axons in transected rat sciatic nerve repaired with epineurial sutures. J Neurol Sci. 1979 Dec;44(1):55-9. https://doi.org/10.1016/0022-510x(79)90222-3
69. Forman DS, Berenberg RA. Regeneration of motor axons in the rat sciatic nerve studied by labeling with axonally transported radioactive proteins. Brain Res. 1978 Nov 10;156(2):213-25. https://doi.org/10.1016/0006-8993(78)90504-8
70. Navarro X, Verdú E, Butí M. Comparison of regenerative and reinnervating capabilities of different functional types of nerve fibers. Exp Neurol. 1994 Oct;129(2):217-24. https://doi.org/10.1006/exnr.1994.1163
71. Tessier-Lavigne M, Goodman CS. The molecular biology of axon guidance. Science. 1996 Nov 15;274(5290):1123-33. https://doi.org/10.1126/science.274.5290.1123
72. Breau MA, Trembleau A. Chemical and mechanical control of axon fasciculation and defasciculation. Semin Cell Dev Biol. 2023 May 15;140:72-81. https://doi.org/10.1016/j.semcdb.2022.06.014