Original article
Ukrainian Neurosurgical Journal. 2024;30(4):43-50
https://doi.org/10.25305/unj.310254
1 Group of Neurophthalmology, Romodanov Neurosurgery Institute, Kyiv, Ukraine
2 Еndonasal Skull Base Surgery, Department Romodanov Neurosurgery Institute, Kyiv, Ukraine
3 Group of Otoneurology, Romodanov Neurosurgery Institute, Kyiv, Ukraine
Received: 20 August 2024
Accepted: 21 October 2024
Address for correspondence:
Valeriia V. Musulevska, Group of Neurophthalmology, Romodanov Neurosurgery Institute, 32 Platona Mayborody st., Kyiv, 04050, Ukraine, e-mail: musulevskaria@gmail.com
Objective: To investigate the effectiveness of radiological methods for diagnosing optic nerve and chiasm atrophy in compressive optic neuropathy caused by tumors of the chiasmal-sellar region (CSR).
Material and methods: The diagnostic and treatment outcomes of 50 patients (100 eyes) with CSR tumors were analyzed. These patients were treated at the A.P. Romodanov Institute of Neurosurgery of the National Academy of Medical Sciences of Ukraine from 2021 to 2023. The study group of patients (50 patients) was divided into two subgroups: Group I – restoration of visual functions (26 patients, 52%, 52 eyes); Group II – no restoration of visual functions (24 patients, 48%, 48 eyes). Clinical-neurological, ophthalmological, and otoneurological examinations were performed. MRI of the brain was conducted on all patients using high-field scanners (1.5 and 3.0 Tesla), and measurements of the optic nerve (ON) diameter in the intraorbital and intracranial parts, as well as the height and width of the chiasm.
Results: The morphometric parameters of the ON diameter in the intraorbital part and the height of the chiasm did not significantly differ between the studied groups (p>0.05). The morphometric parameters of Group I did not differ from the control group (p>0.05). In Group II the average diameter of the intracranial part of the ON (2.31±0.26 mm) and the average width of the chiasm (11.39±0.31 mm) were statistically significantly different from the control group values: 2.97±0.2 mm and 13.69±0.57 mm, respectively, p<0.05. Despite significant variability in individual characteristics, the parameters of the intracranial part of the ON ≤ 2.31 mm and the chiasm width of ≤ 11.39 mm indicate irreversible atrophic changes and can be used to predict ophthalmological outcomes in patients with CSR tumors.
Conclusions: Measuring the thickness of the chiasm and the diameter of the intracranial part of the optic nerve using high-resolution MRI is a convenient and effective method for diagnosing optic nerve atrophy (ONA) and predicting ophthalmological outcomes after decompression of the optochiasmal complex.
Keywords: chiasmal-sellar region tumors; high-resolution MRI; compressive optic neuropathy; optic nerve atrophy
Introduction
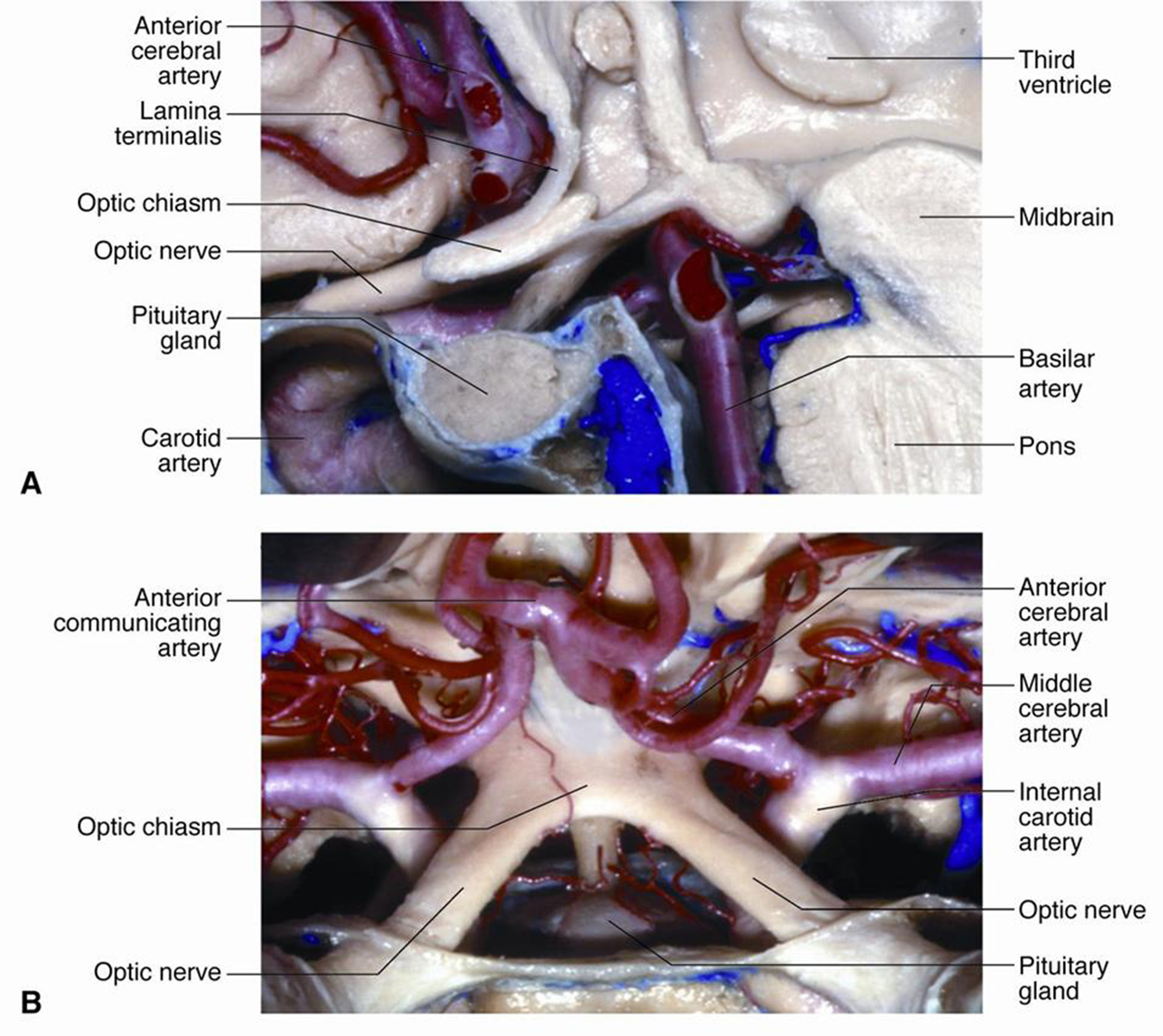
The optic chiasm is a crucial neuroanatomical structure in the brain where the optic nerves converge and partially cross. It is located above the area of the tuberculum sellae. Above the chiasm lies the floor of the third ventricle with the recessus opticus, as well as the anterior cerebral and anterior communicating arteries, while below, it borders the diaphragm of the tuberculum sellae. The internal carotid arteries are located laterally to the chiasm [1-3] (Fig. 1).

Fig. 1. Anatomy of the chiasmal-sellar region: A – sagittal section; B – horizontal section. The intracranial part of the optic nerves is shown [1]
The total length of the optic nerve (ON) ranges from 35 to 55 mm. It consists of intraorbital, intracanalicular, and intracranial sections. The intracranial section is the most variable, measuring 4–17 mm [4]. Studies using histological methods and magnetic resonance imaging (MRI) at 1.5 Tesla have shown a reduction in the normal diameter of the ON along the intraorbital segment from (3.99±0.04) mm immediately behind the eyeball to (3.50±0.04) mm at a distance of 10 mm from the posterior pole of the eyeball [5]. S. Mncube and M. Goodier (2019) conducted a study using high-resolution MRI (>1.5 Tesla) and reported a range of normal ON diameters: the intraorbital section at 5 mm from the posterior pole of the eyeball measured 2.52 mm (1.53–3.69 mm), at 10 mm from the posterior pole 2.37 mm (1.44–3.63 mm), and the intracranial section 4.27 mm (2.46–5.19 mm) [6]. According to M. Prairie et al. (2024), an ON diameter of ≤2.3 mm at 10 mm from the posterior pole of the eyeball is a highly sensitive and specific indicator of optic nerve atrophy (ONA) [7]. B. Zhao et al. (2019) found that an ON area of ≤4.0 mm² measured by MRI is both highly sensitive and specific for predicting the presence of ONA [8].
Studies using high-resolution MRI have reported that the average area of the chiasm is between 27.07 and 43.7 mm², with an average length of 5–12 mm, average width of 12.23–15.0 mm, and average height of 1.93–3.5 mm [6, 9, 10]. According to J. Parravano et al. (1993), a chiasm width of <13.5 mm is a marker of optic nerve atrophy (ONA) [11].
The morphometric parameters of the optic nerve (ON) and chiasm reported in the literature vary, but most researchers agree that the width of the chiasm and the diameter of the intracranial portion of the ON are reliable measurement indicators [6].
Assessing the morphometric parameters of the ON and chiasm with MRI can provide additional information for diagnosing ONA and predicting ophthalmological outcomes following the removal of tumors in the chiasmal-sellar region (CSR). However, there are few reports on average chiasm sizes based on MRI in the healthy population, and existing data are inconsistent. Given the individual variability in chiasm size and ON thickness, identifying parameters that could supplement objective ophthalmological data on atrophic changes in the visual pathway is relevant.
Objective: To analyze the effectiveness of radiological methods for diagnosing optic nerve and chiasm atrophy in compressive optic neuropathy caused by tumors in the chiasmal-sellar region.
Materials and Methods
Study participants
The diagnostic and treatment outcomes of 50 patients (100 eyes) with chiasmal syndrome caused by CSR tumors—pituitary adenomas in 26 patients (52%) and tuberculum sellae meningiomas in 24 patients (48%)—were analyzed. These patients received treatment at the A.P. Romodanov Institute of Neurosurgery, National Academy of Medical Sciences of Ukraine, from 2021 to 2023 (study group). The study group included 25 women (50%) and 25 men (50%), with an average age of 50.50±10.21 years.
The study was conducted in compliance with bioethics principles, including the provisions of the Declaration of Helsinki on human rights, and was approved by the Ethics Committee of the A.P. Romodanov Institute of Neurosurgery, National Academy of Medical Sciences of Ukraine (minutes No. 5, dated 13.12.2019). All patients provided informed and voluntary written consent to participate in the study and for data publication.
Inclusion criteria
The inclusion criteria were: pituitary adenomas and tuberculum sellae meningiomas without optic nerve canal involvement, presence of chiasmal syndrome, and a follow-up period of at least 12 months.
Exclusion criteria included progressive tumor growth, supradiaphragmatic craniopharyngiomas and other CSR tumors with cystic components, patients with signs of intracranial hypertension, prior radiation therapy or surgery, and concurrent ophthalmological diseases. Craniopharyngiomas were excluded from the study as the visual impairment in these cases is caused not only by optic nerve atrophy (ONA) but also by tumor invasion into the ON and/or chiasm. Assessing the extent of visual function restoration is challenging due to potential intraoperative devascularization of the visual apparatus, which significantly affects postoperative visual acuity.
Group characteristics
Based on ophthalmological outcomes following CSR tumor removal, patients were divided into two subgroups: Group I, with restoration of visual functions (26 patients, 52%, 52 eyes), and Group II, without restoration of visual functions (24 patients, 48%, 48 eyes). The control group consisted of 20 healthy adults (40 eyes) with no ophthalmological or neurosurgical pathology.
Study Design
The patients underwent clinical-neurological, ophthalmological, and otoneurological examinations using instrumental and laboratory diagnostic methods.
All patients underwent MRI of the brain using high-field scanners (1.5 and 3.0 Tesla) in native mode and with contrast enhancement in three projections. Standard brain imaging protocols included T1WI and T2WI T2WI slices. MRI was performed at least two weeks prior to surgery. The ON diameter was measured at two points: the intraorbital section (10 mm from the posterior pole of the eyeball) and the intracranial section (5–6 mm from the chiasm) in the axial projection (Fig. 2). The height of the chiasm was measured in the frontal (coronal) projection, and the width of the chiasm in the axial projection (Fig. 3).
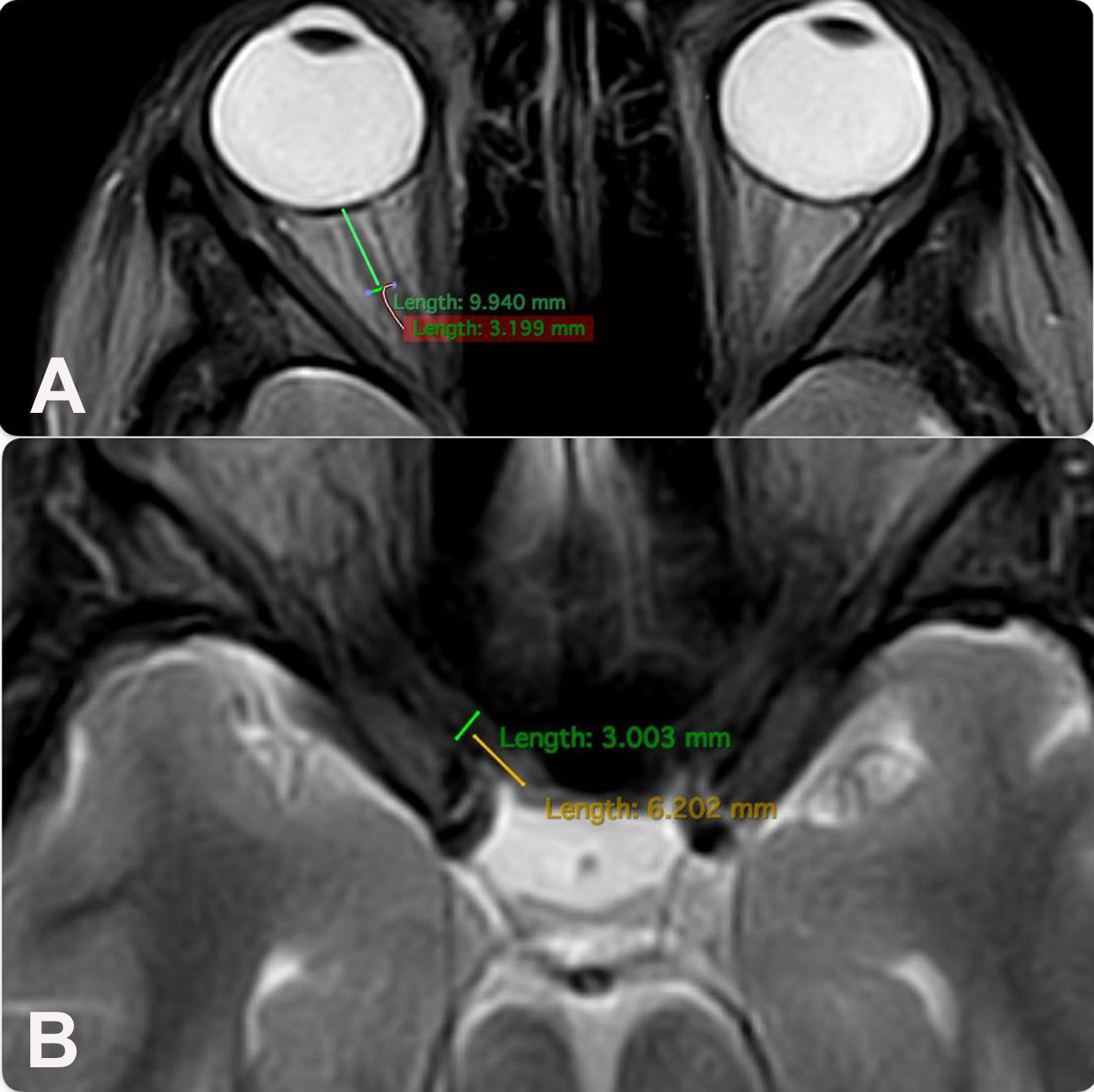
Fig. 2. Technique for measuring the diameter of the optic nerve (brain MRI, axial projection, T1-weighted images):
A – intraorbital part;
B – intracranial part
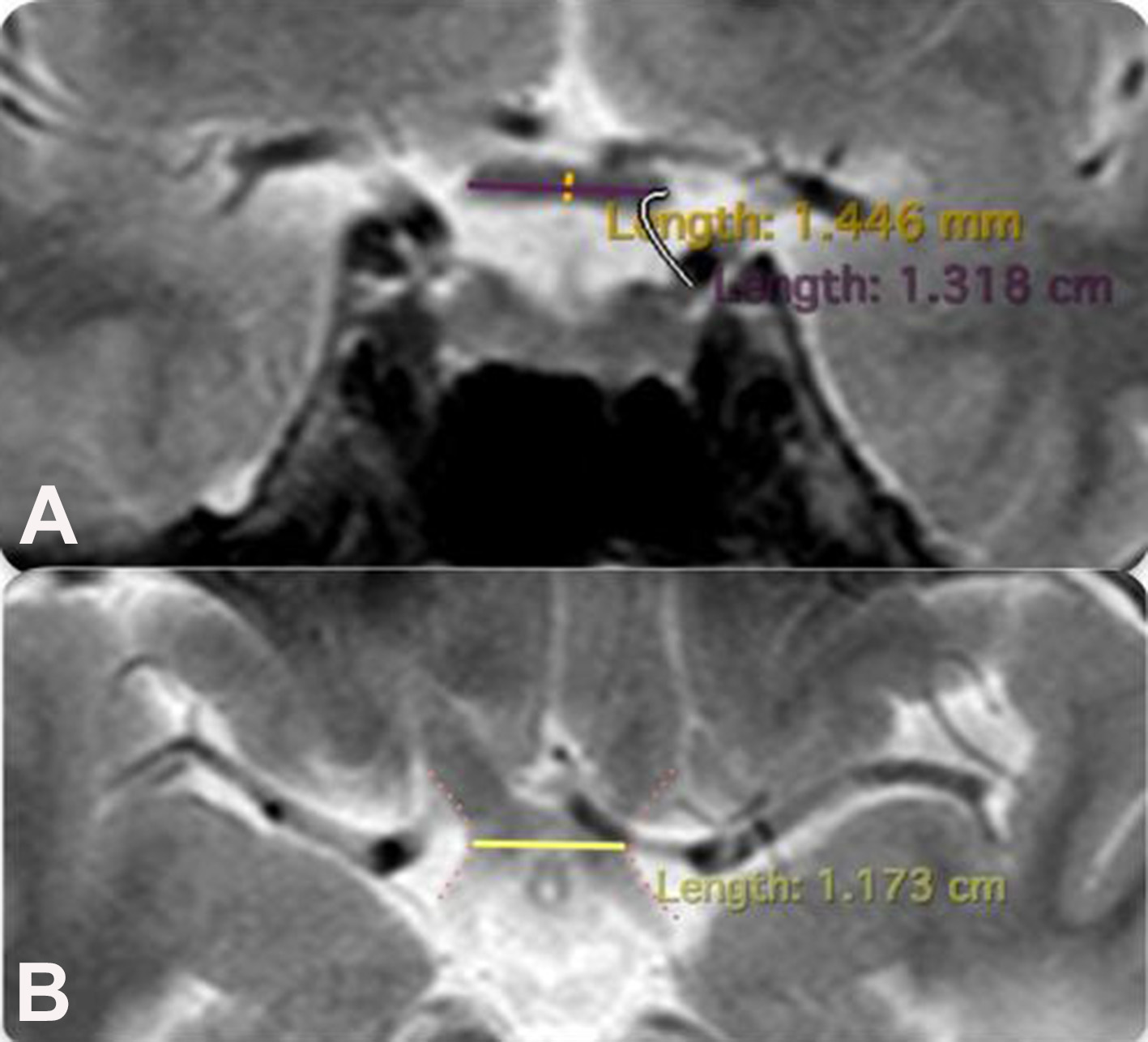
Fig. 3. Technique for measuring chiasm parameters:
A – height (brain MRI, coronal projection T1WI-weighted images), marked by a yellow line;
B – width (brain MRI, axial projection T1WI-weighted images), marked by a yellow line
The ophthalmological examination included visometry, biomicroscopy, perimetry (both kinetic and static), and ophthalmoscopy (both direct and indirect). The first examination was performed on the 1st–2nd day after hospital admission, the second on the 5th–7th day of the postoperative period (early postoperative period), and follow-ups were conducted at 1, 3, and 6 months, as well as at 1 year. Examinations continued throughout the year if delayed (late) visual function recovery was observed.
Visual acuity was tested with optimal correction. The visual field was assessed using the Centerfield 2 perimeter (Germany) with the "Threshold test neuro - 30-2" and "Neuro screening" programs, focusing on defect localization and the mean deviation (MD) indicator of total light sensitivity loss.
Visual function recovery was defined as an improvement in visual acuity to ≥1.0, elimination of visual field defects, and MD < -2 dB in both eyes.
Statistical Analysis
Data were entered into Excel and analyzed using the "Statistica 6.0" software. Results are presented as the mean ± standard deviation (M±SD). To assess the significance of differences (p-value) between independent groups, Student's t-test for paired samples was applied. A p-value < 0.05 was considered statistically significant. Pearson’s χ² test or Fisher's exact test was used to evaluate the distribution frequency for traits when the sample size was small.
Results and Discussion
The results of the morphometric measurements of the ON and chiasm are presented in Tables 1 and 2.
Table 1. Clinical and morphometric characteristics of the study groups
|
Clinical data |
Group I |
Group II |
Student’s t-test value, p |
|
Age, years |
51,50±11,38 |
50,4±9,5 |
t=0,07 |
|
Visual Acuity |
0,66±0,31 |
0,55±0,32 |
t=0,25 |
|
MD, dB |
13,81±0,82 |
15,32±0,71 |
t=1,39 |
|
Disease duration, months |
5,35±4,38 |
21,80±0,32 |
t=3,75* |
|
Diameter of intraorbital part of ON, mm |
2,99±0,16 |
2,88±0,26 |
t=0,36 |
|
Diameter of intracranial part of ON, mm |
2,84±0,25 |
2,31±0,26 |
t=0,64 |
|
Chiasm Height, mm |
2,13±0,35 |
1,97±0,24 |
t=0,38 |
|
Chiasm Width, mm |
12,86±0,32 |
11,39±0,31 |
t=3,3* |
Note: * – The difference in values is statistically significant.
Table 2. Average morphometric parameters of the optic nerve and chiasm, mm
|
Group |
Diameter of intraorbital part of ON |
Diameter of intracranial part of ON |
Chiasm height |
Chiasm width |
|
Control Group, n=20 |
3,08±0,25 |
2,97±0,20 |
2,01±0,35 |
13,69±0,57 |
|
Group I, n=26 |
2,99±0,16 |
2,84±0,25 |
2,13±0,35 |
12,86±0,32 |
|
Group II, n=24 |
2,88±0,26 |
2,31±0,26* |
1,97±0,24 |
11,39±0,31* |
Note: * – The difference in values is statistically significant.
A uniform age distribution was found across both groups (p > 0.05). Patients in the study groups demonstrated reduced visual acuity and visual field defects in the form of bitemporal heteronymous hemianopia (complete or partial) (Figures 4 and 5).
A 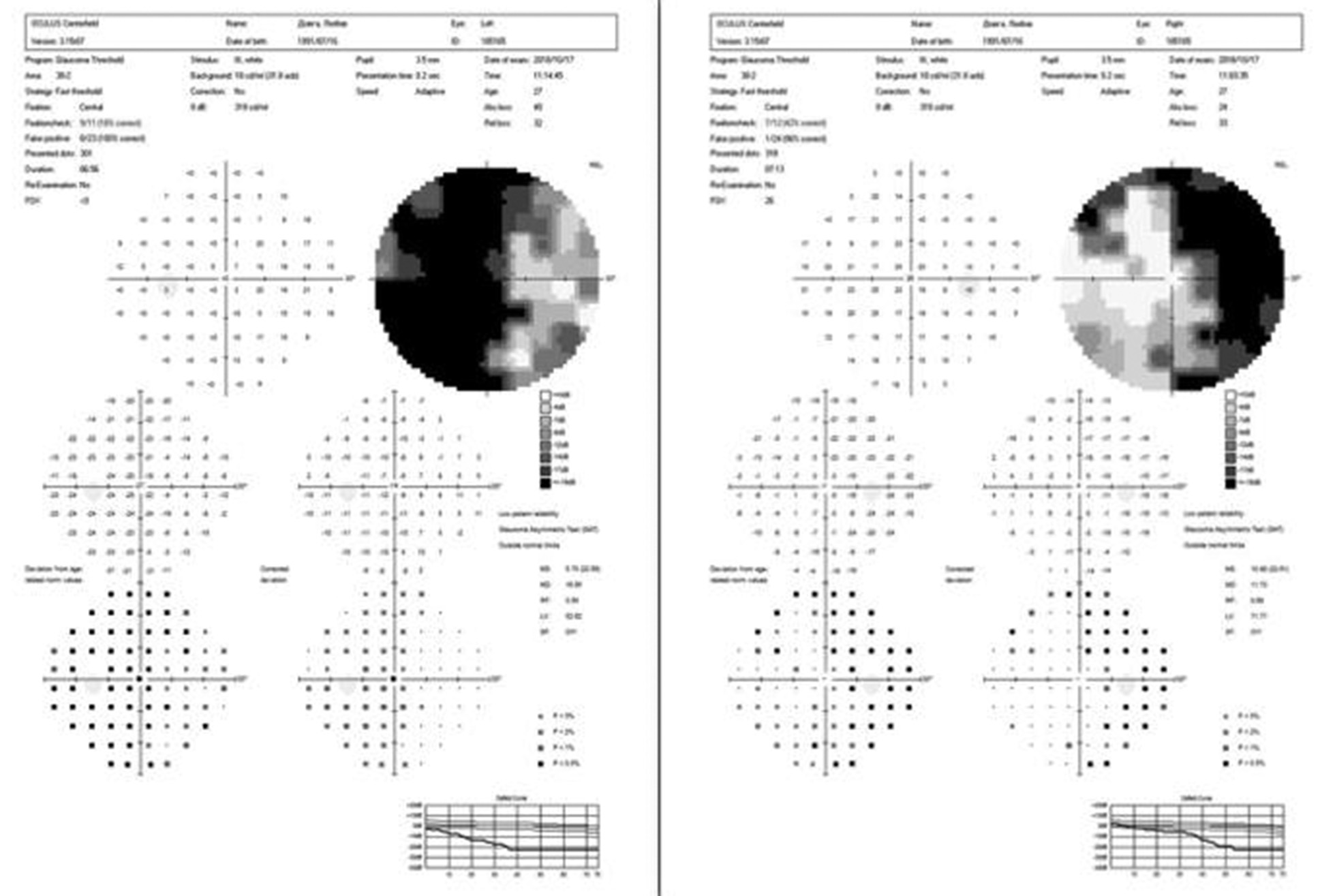
B 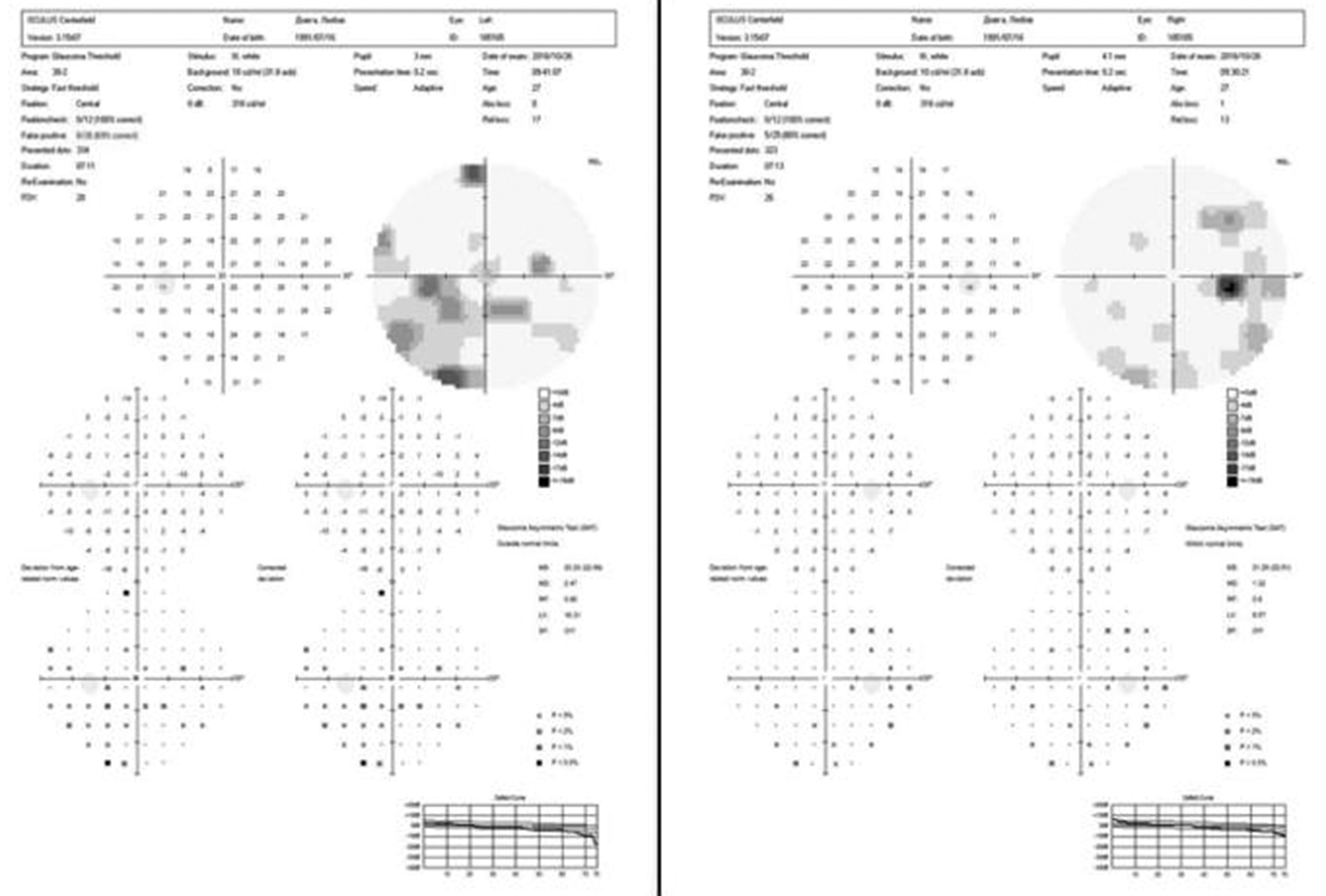
Fig. 4. Automated static perimetry of a patient from group I: A – before surgery; B – after surgery
A 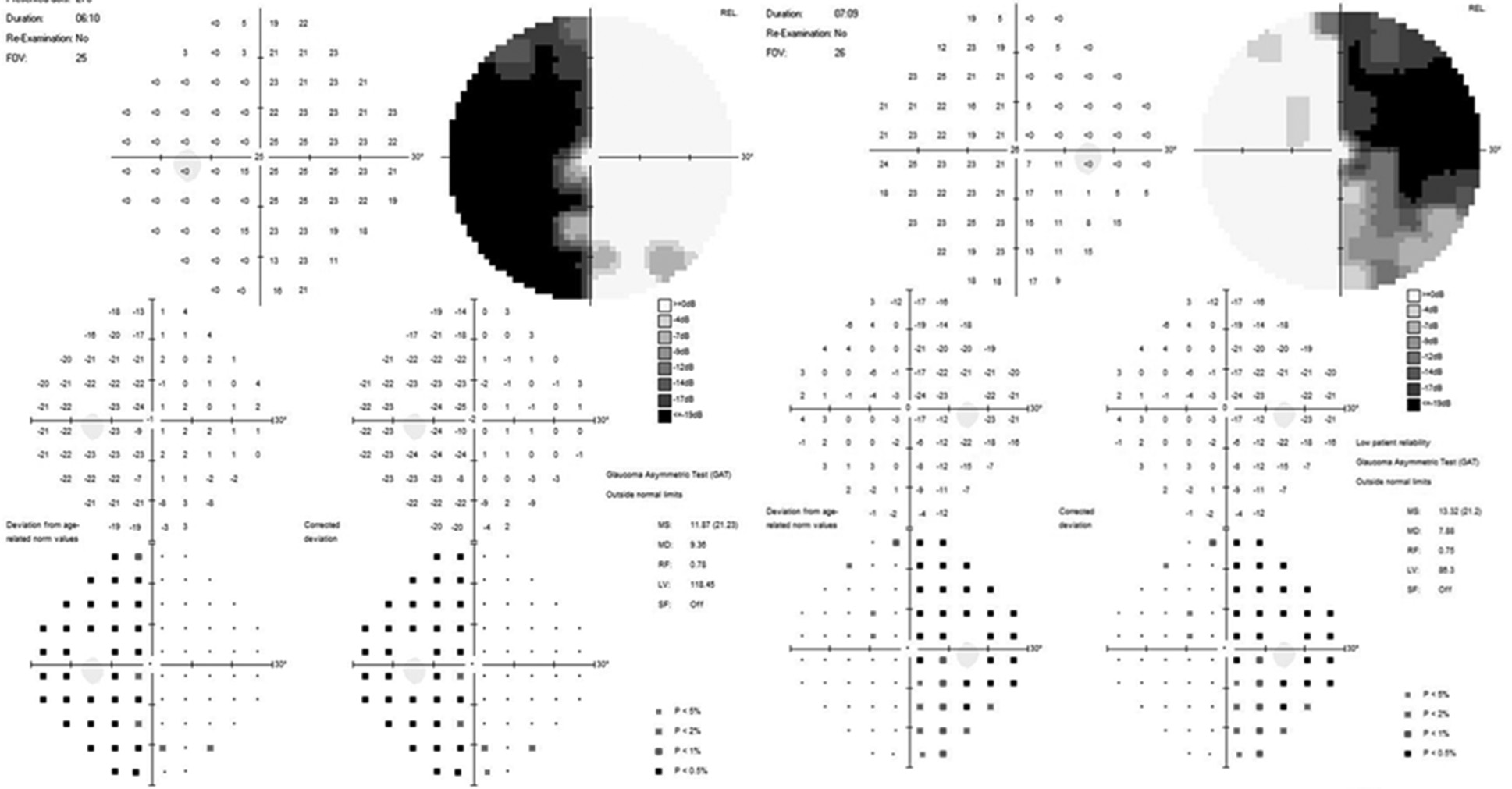
B 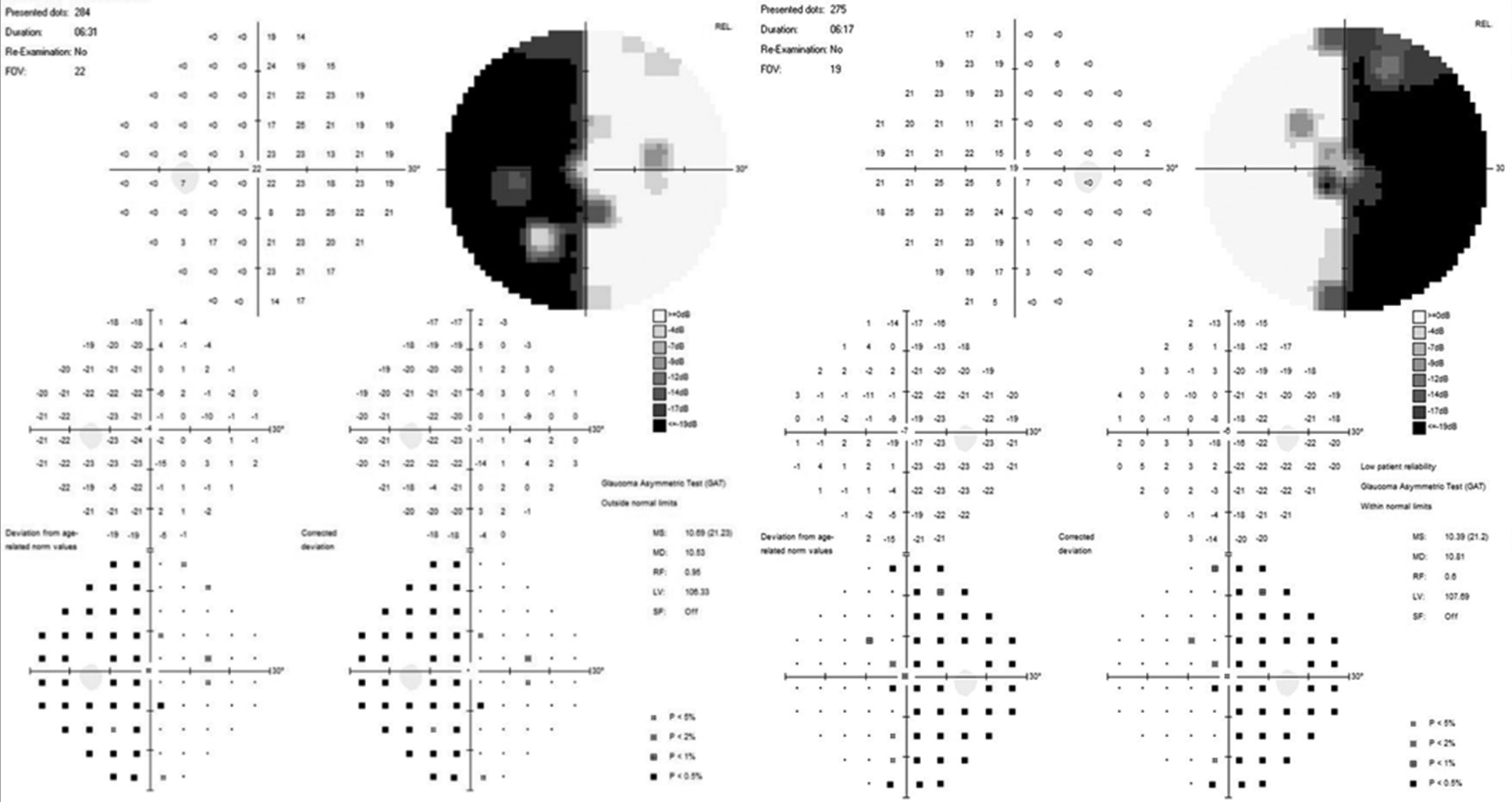
Fig. 5. Automated static perimetry of a patient from Group II: A – before surgery; B – after surgery.
It was established that the average visual acuity values (Group I – 0.66±0.31, Group II – 0.55±0.32) and the average total light sensitivity loss before surgery (group I – 13.81±0.82 dB, group II – 15.32±0.71 dB) did not differ significantly (p>0.05). However, the duration of visual impairments was significantly different (Group I – 5.35±4.38 months, Group II – 21.80±0.32 months, p<0.05).
The diameter of the ON in the intraorbital part and the chiasm height did not differ significantly between groups (p>0.05). There was also no statistically significant difference in morphometric parameters between group I and the control group (p>0.05). In group II, the average diameter of the intracranial part of the optic nerve (2.31±0.26 mm) and the average chiasm width (11.39±0.31 mm) were significantly smaller than in the control group (2.97±0.20 mm and 13.69±0.57 mm, respectively, p<0.05). The diameter of the intraorbital part of the optic nerve and the chiasm height in group II tended to decrease, though the difference was not statistically significant (p>0.05).
In healthy individuals, the average optic nerve diameter was: intraorbital part – 3.08±0.25 mm, intracranial part – 2.97±0.20 mm, consistent with values reported by S.S. Mncube and M.D. Goodier (2019) [6], at 2.52 mm (1.53–3.69 mm) and 4.27 mm (2.46–5.19 mm), and the average chiasm width (13.69±0.57 mm) aligns with findings from S.S. Mncube and M.D. Goodier (2019), – 13.63 mm (11.13–16.92 mm), and is only slightly different from values reported by S.O. Polat et al. (2020) – 12.82±1.27 mm and V. Juenger et al. (2020) – 12.23±1.15 mm [9, 10].
According to the literature [6, 9, 10], the chiasm height in healthy individuals varies from 1.93 to 3.5 mm, which is consistent with our findings of 2.01±0.35 mm. The lack of a statistically significant difference in chiasm height across the studied groups suggests low diagnostic value for this parameter in diagnosing optic nerve atrophy (ONA), likely due to the difficulty of measuring small structures under chiasmal compression.
An intracranial optic nerve diameter of ≤2.31 mm and a chiasm width of ≤11.39 mm indicate irreversible atrophic changes and may be used to predict ophthalmic outcomes in patients with skull base tumors. These findings align with the conclusion by M. Prairie et al. (2024) that an intracranial optic nerve diameter of ≤2.3 mm is a highly sensitive and specific marker for ONA [7]. According to J. Parravano et al. (1993), a chiasm width of <13.5 mm is a sign of ONA. Such conflicting conclusions may stem from technical challenges in using low-resolution MRI to measure small structures in the visual pathway [11].
The results obtained suggest that a reduction in chiasm width and intracranial optic nerve diameter occurs earlier than reductions in chiasm height and the diameter of the intraorbital part of the ON.
Conclusions
High-resolution MRI enables informative visualization of the structures of the visual pathway and the characteristics of CSR.
In cases of compressive optic neuropathy, an intracranial optic nerve diameter of ≤2.31 mm and a chiasm width of ≤11.39 mm indicate atrophic changes, which can serve as a convenient supplementary diagnostic tool for optic nerve atrophy (ONA). These indicators can be used to predict visual recovery outcomes in the surgical treatment of certain chiasmal-sellar region.
Disclosure
Conflict of Interest
The authors declare no conflict of interest.
Ethical Standards
All procedures performed on patients during the study adhered to the ethical standards of the institutional and national ethics committees and to the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Informed Consent
Informed consent was obtained from all patients.
Funding
The study received no sponsorship or financial support.
References1. Rhoton AL Jr. The sellar region. Neurosurgery. 2002 Oct;51(4 Suppl):S335-74. https://doi.org/10.1097/00006123-200210001-00009
2. Danesh-Meyer HV, Yoon JJ, Lawlor M, Savino PJ. Visual loss and recovery in chiasmal compression. Prog Retin Eye Res. 2019 Nov;73:100765. https://doi.org/10.1016/j.preteyeres.2019.06.001
3. Gupta M, Ireland AC, Bordoni B. Neuroanatomy, Visual Pathway. 2022 Dec 19. In: StatPearls. Treasure Island (FL): StatPearls Publishing; 2024 Jan–.
4. Kiernan JA, Barr ML. Barr's the human nervous system: an anatomical viewpoint. Lippincott Williams & Wilkins; 2014.
5. Karim S, Clark RA, Poukens V, Demer JL. Demonstration of systematic variation in human intraorbital optic nerve size by quantitative magnetic resonance imaging and histology. Invest Ophthalmol Vis Sci. 2004 Apr;45(4):1047-51. https://doi.org/10.1167/iovs.03-1246
6. Mncube SS, Goodier MD. Normal measurements of the optic nerve, optic nerve sheath and optic chiasm in the adult population. SA J Radiol. 2019 Nov 5;23(1):1772. https://doi.org/10.4102/sajr.v23i1.1772
7. Prairie ML, Gencturk M, McClelland CM, Marka NA, Jiang Z, Folkertsma M, Lee MS. Establishing Optic Nerve Diameter Threshold Sensitive and Specific for Optic Atrophy Diagnosis. Clin Neuroradiol. 2024 Jun;34(2):373-378. https://doi.org/10.1007/s00062-023-01369-w
8. Zhao B, Torun N, Elsayed M, Cheng AD, Brook A, Chang YM, Bhadelia RA. Diagnostic Utility of Optic Nerve Measurements with MRI in Patients with Optic Nerve Atrophy. AJNR Am J Neuroradiol. 2019 Mar;40(3):558-561. https://doi.org/10.3174/ajnr.A5975
9. Polat SÖ, Öksüzler FY, Öksüzler M, Uygur AG, Yücel AH. The determination of the pituitary gland, optic chiasm, and intercavernous distance measurements in healthy subjects according to age and gender. Folia Morphol (Warsz). 2020;79(1):28-35. https://doi.org/10.5603/FM.a2019.0058
10. Juenger V, Cooper G, Chien C, Chikermane M, Oertel FC, Zimmermann H, Ruprecht K, Jarius S, Siebert N, Kuchling J, Papadopoulou A, Asseyer S, Bellmann-Strobl J, Paul F, Brandt AU, Scheel M. Optic chiasm measurements may be useful markers of anterior optic pathway degeneration in neuromyelitis optica spectrum disorders. Eur Radiol. 2020 Sep;30(9):5048-5058. https://doi.org/10.1007/s00330-020-06859-w
11. Parravano JG, Toledo A, Kucharczyk W. Dimensions of the optic nerves, chiasm, and tracts: MR quantitative comparison between patients with optic atrophy and normals. J Comput Assist Tomogr. 1993 Sep-Oct;17(5):688-90. https://doi.org/10.1097/00004728-199309000-00003