Original article
Ukrainian Neurosurgical Journal. 2024;30(4):23-29
https://doi.org/10.25305/unj.309045
Department of Neurology and Neurosurgery, Odesa National Medical University, Odesa, Ukraine
Received: 25 July 2024
Accepted: 02 September 2024
Address for correspondence:
Yuliia O. Solodovnikova, Department of Neurology and Neurosurgery, Odesa National Medical University, 8 Tinysta Street, Odesa , 65125, Ukraine, e-mail: julie-sinel@ukr.net
Objective to determine the effect of the number of multiple intracranial aneurysms (MIA) on the course of the acute period of subarachnoid hemorrhage (SAH).
Materials and methods: A cross-sectional retrospective study was conducted involving 92 patients in the acute phase of SAH due to the rupture of MIA. Patients were divided into two groups depending on the number of aneurysms. Demographic indicators (age, gender) and clinical-instrumental factors (comorbidities, extent of hemorrhage, severity at admission, clinical manifestations), treatment characteristics, and outcomes were analyzed using descriptive statistics and logistic regression.
Results: Comorbidities were 3.4 times more common among patients with three or more aneurysms (p=0.143). Patients in group 2 were 1.9 times more likely to be admitted with a World Federation of Neurosurgical Societies (WFNS) grade 2 (p=0.335). WFNS grade 3 at admission was observed twice as often in group 1 (p=0.447). Patients from group 1 and group 2 were equally likely to present with WFNS grade 4 (p=0.978). The probability of being admitted with a WFNS grade 5 was 1.8 times higher in group 2 (p=0.830). The presence of meningeal syndrome increased the risk of having 3 or more aneurysms by more than four times (OR 4.41, CI 0.41-47.13, p=0.21). The presence of motor impairments significantly reduced the risk of having 3 or more aneurysms (OR 0.63, CI 0.09-4.18, p=0.63). Patients in group 2 were slightly more likely to develop vasospasm than those in group 1 (OR 1.22, CI 0.34-4.31, p=0.752). The presence of comorbidities increased with the number of aneurysms (OR 3.42, CI 0.65-17.62, p=0.143).
Conclusions: The presence of comorbidities more than triples the chances of having 3 or more aneurysms. Patients with fewer aneurysms are twice as likely to be admitted in a milder condition (WFNS grade 2). The probability of severe condition (WFNS grade 5) at hospital admission for patients in group 2 is 1.8 times higher than in group 1. The likelihood of motor disorders decreases by 4.4 times with an increasing number of aneurysms. The probability of vasospasm development slightly increased with the number of aneurysms. These data emphasize the importance of a comprehensive approach to the assessment of SAH patients and the need for careful monitoring of patients at high risk of vasospasm.
Key words: subarachnoid hemorrhage; multiple intracranial aneurysms; clinical course
Introduction
Multiple intracranial aneurysms (MIA) account for 20–34% of all intracranial aneurysms and carry a higher risk of rupture compared to solitary aneurysms. Key risk factors for rupture include age, sex, and aneurysm location. The annual rupture risk for multiple aneurysms is 1–2%, depending on additional factors such as hypertension and a personal or family history of hemorrhages [1, 2].
Recent studies have identified complex genetic and molecular mechanisms influencing MIA rupture [3]. Inherited genetic mutations are often associated with an increased risk of aneurysm development and rupture. Conditions such as Ehlers-Danlos and Marfan syndromes, as well as Factor VII deficiency, are known to compromise vascular wall integrity, increasing the likelihood of MIA formation [4].
Molecular mechanisms involving mitochondrial protein dysfunction also play a significant role in the pathogenesis of MIA rupture. Proteins such as AIF1, CCDC90B, and tRNA PusA are linked to an elevated rupture risk due to their influence on cellular energy metabolism and structural processes in vascular walls [5]. Inflammatory processes caused by immune cell dysfunction can promote the development of MIA, particularly in the presence of hypertension [4].
Multiple cerebral aneurysms are associated with a higher risk of complications, especially rupture, compared to solitary aneurysms. The rupture risk varies depending on the aneurysm's location, size, and morphology. In our study, the size of ruptured aneurysms in most cases ranged from 5 to 10 mm, but this factor needs further investigation because of differences between studies. Aneurysms located in the anterior communicating artery ruptured more frequently, while fewer ruptures were observed in the internal carotid and middle cerebral arteries. Ruptures were observed only in patients with two or three aneurysms, with the highest rupture frequency in the MIA group [6].
Aneurysms in the posterior cerebral circulation or with a diameter exceeding 5 mm were more prone to rupture. Additionally, patients with a history of aneurysmal subarachnoid hemorrhage (SAH) faced an increased risk of rebleeding from another aneurysm [1, 2].
The course of MIA presents several challenges for diagnosis and treatment. Often asymptomatic, these aneurysms may only become clinically evident upon rupture, resulting in life-threatening conditions such as SAH. While symptoms like severe headache, nausea, loss of consciousness, or stroke-like presentations may occur after rupture, early signs are often minimal or absent. MIAs can vary in size and location, with some remaining stable while others grow or rupture due to factors like hypertension or additional risk conditions. Monitoring is complicated as each aneurysm carries a unique rupture risk [4].
When MIAs are located in different vascular regions, complications such as recurrent hemorrhages can occur even after surgical intervention on one aneurysm. For example, following treatment of one aneurysm, another may remain undetected or form anew at another site [5]. Studies show that MIA patients are at risk of developing new (de novo) aneurysms, underscoring the need for continuous monitoring, particularly after surgical treatment. Contributing factors include hypertension, atherosclerosis, smoking, and genetic predispositions affecting vascular wall stability [4].
Hypertension is a major risk factor for aneurysm rupture, as increased arterial pressure can weaken vessel walls already compromised by aneurysms. Patients with hypertension must closely monitor their blood pressure to mitigate this risk [2, 7].
A multivariate analysis of the risk of MIA rupture revealed a particularly negative impact of age under 40, smoking more than 20 cigarettes per day, uncontrolled hypertension, and aneurysm location on the C7 segment of the internal carotid artery or anterior communicating artery. Ruptured aneurysms tend to be larger and had a wider neck [8].
Atherosclerotic changes reduce vascular elasticity, increasing rupture susceptibility under hemodynamic stress [1].
While evidence on the direct impact of diabetes on aneurysm rupture is conflicting, it is believed that diabetes may worsen the overall health of the vessels and reduce their ability to recover from damage, indirectly increasing the risk of rupture. Smoking-related toxins damage vessel walls, promoting aneurysm formation and rupture. Smoking is also associated with the development of new aneurysms and its rupture [2].
Objective: To analyze the impact of the number of arterial aneurysms on the acute phase of subarachnoid hemorrhage.
Materials and Methods
Study Participants
The study analyzed 480 medical records of patients who underwent inpatient treatment at Odesa City Clinical Hospital No. 11 between 2000 and 2023, of whom 92 were diagnosed with multiple intracranial aneurysms (MIA).
The study was approved by the ethics and bioethics committee of Odesa National Medical University (minutes No. 7, dated September 30, 2019). All patients provided informed and voluntary written consent to participate in the study and for the publication of their data.
Inclusion Criteria
Patients in the acute phase of subarachnoid hemorrhage (SAH) caused by the rupture of MIA.
Group Characteristics
Patients with ruptured MIA were divided into two groups based on the number of aneurysms: Group 1 (n = 71): Patients with two arterial aneurysms (AA), Group 2 (n = 21): Patients with three or more arterial aneurysms (≥3 AA).
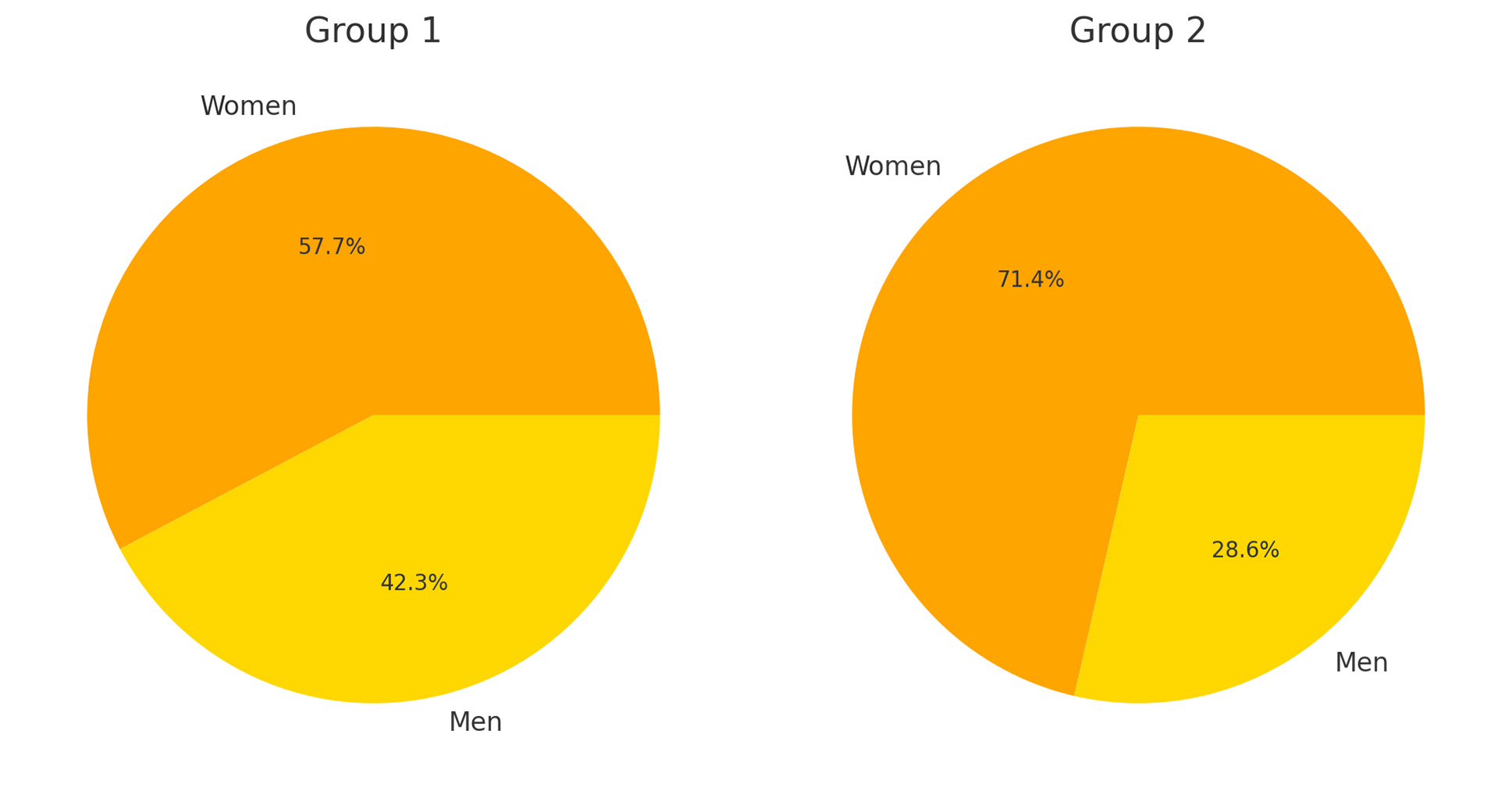
The groups were comparable in terms of age (Fig. 1). However, women were significantly more prevalent in Group 2 (Fig. 2).

Fig. 1. Gender distribution
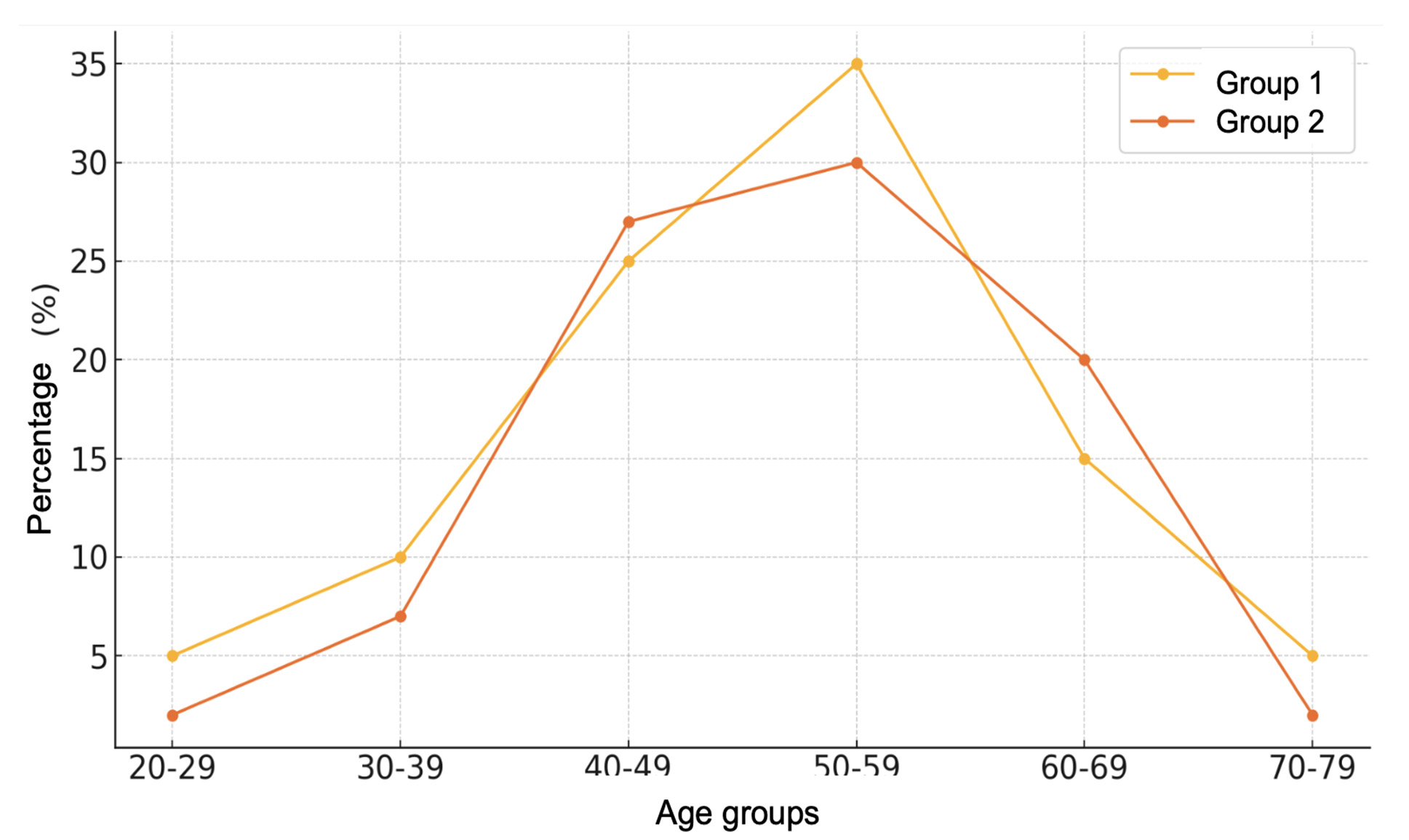
Fig. 2. Age distribution
In Group 2, the number of patients with 3 AAs was 16 patients (76.2%), 4 AAs - 4 (19%), 5 AAs - 1 (2.4%) and 6 AAs - 1 (2.4%)
Most patients with MIA were admitted in a mild condition, with preserved or only slightly impaired consciousness. Only 2.2% of patients were admitted in a critical condition. Differences in severity between the groups were observed: the proportion of patients with ≥3 AAs admitted in critical condition was significantly higher (Fig. 3).
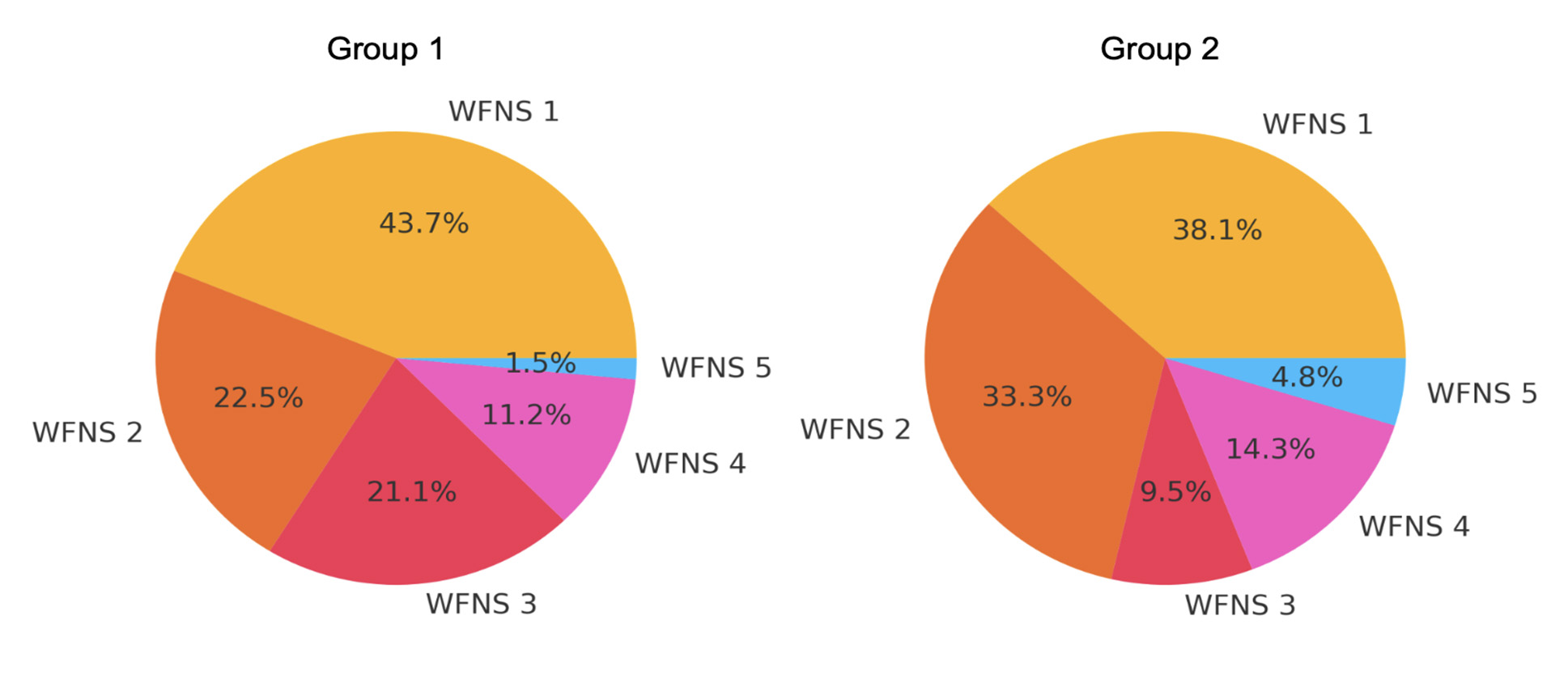
Fig. 3. Distribution of patients by WFNS scale at admission
Study Design
A cross-sectional, retrospective study was conducted. The analysis included demographic factors (age, gender), clinical and instrumental features of SAH progression (comorbidities, hemorrhage extent, severity at admission, clinical manifestations), treatment specifics, and outcomes.
Statistical Analysis
Descriptive statistics and logistic regression were used in the study. A significance threshold (p-value) of 0.05 was applied for hypothesis testing. All calculations were performed using Microsoft Excel.
Results and Discussion
Positive meningeal signs were identified in 88% of patients with MIA. No statistically significant differences were observed between the groups (Fig. 4).
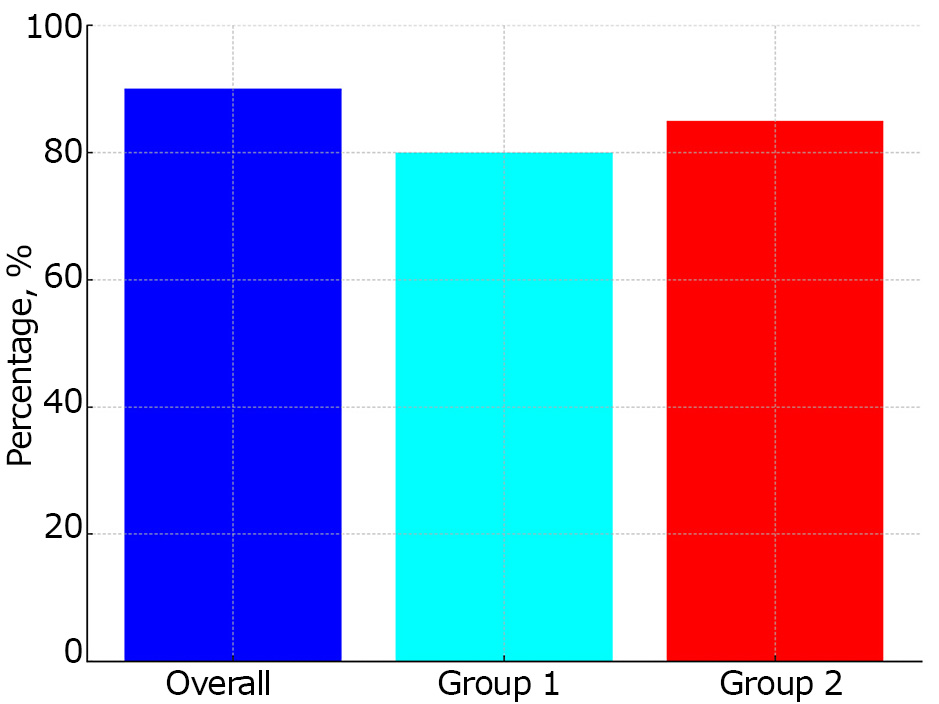
Fig. 4. Meningeal syndrome
Motor deficits at admission varied significantly between the groups. Paresis was observed in 77 patients (84%) in Group 1 and in only 2 patients (9.5%) in Group 2.
Vasospasm was detected in 47 patients (51.1%). The mortality rate among patients with vasospasm was 11.1%, compared to 19.1% in patients without vasospasm.
The patterns of hemorrhage distribution in patients with MIA are shown in Fig. 5 and 6.
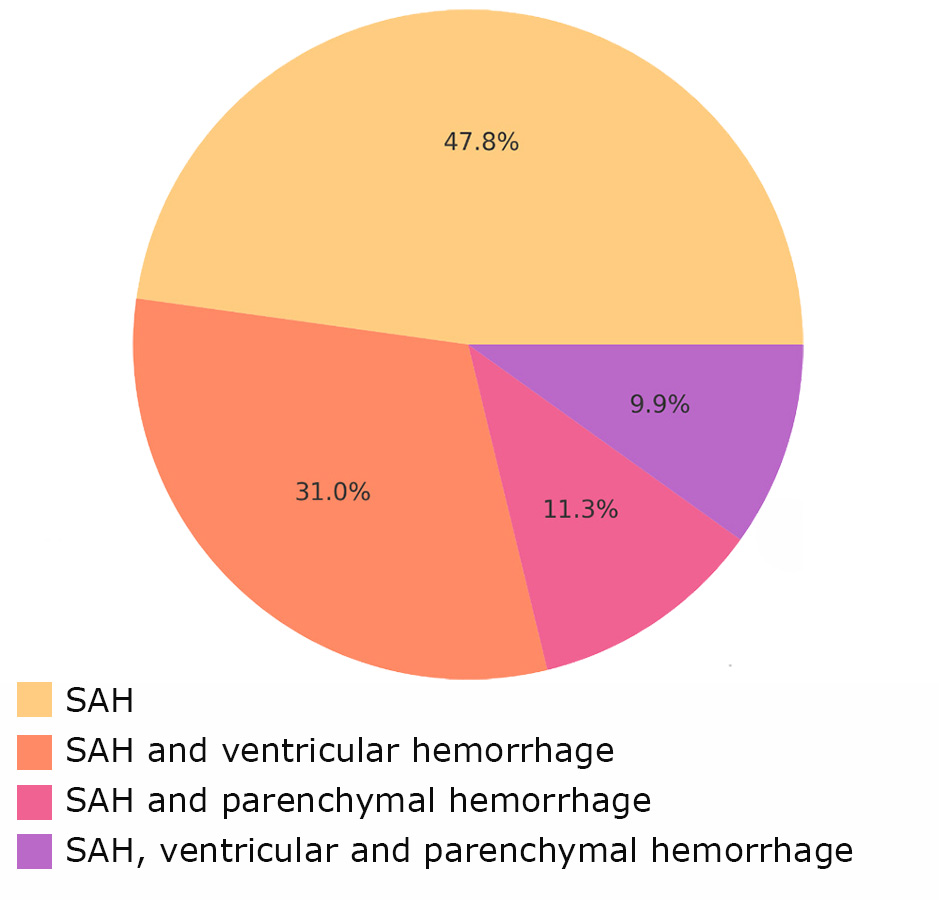
Fig. 5. Types of hemorrhage in Group 1
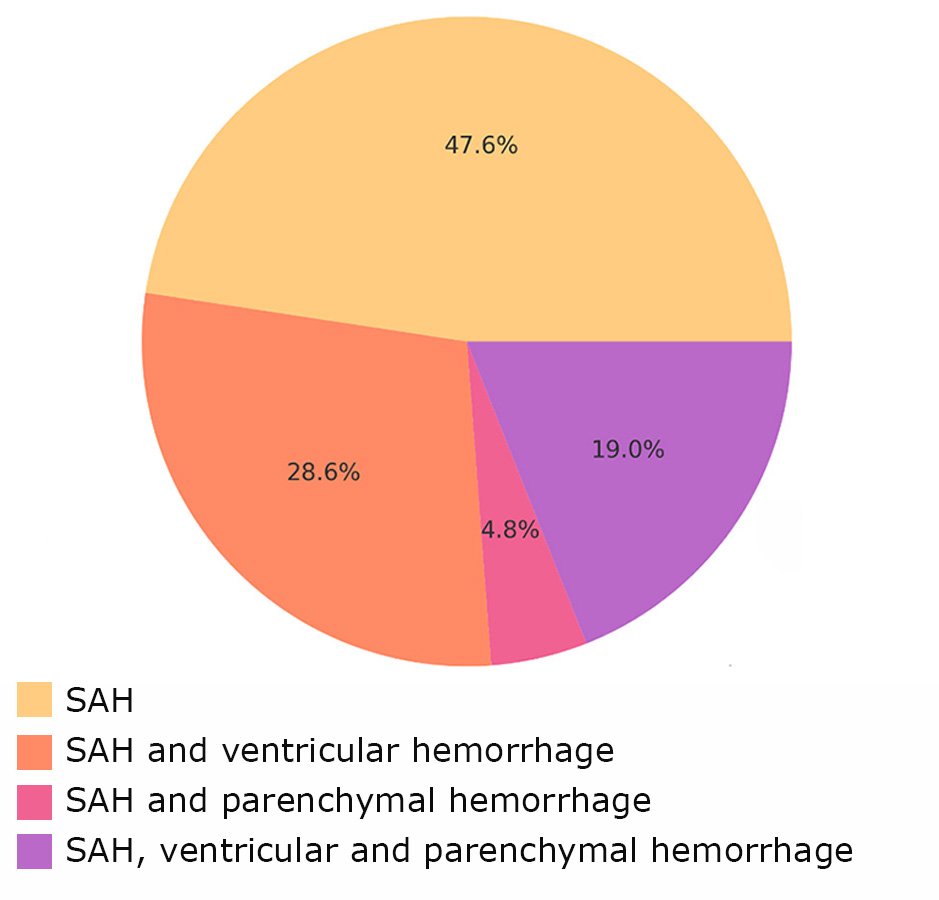
Fig. 6. Types of hemorrhage in Group 2
Intracranial complications were more frequent in Group 1, whereas extracranial septic complications were predominant in Group 2 (Fig. 7).
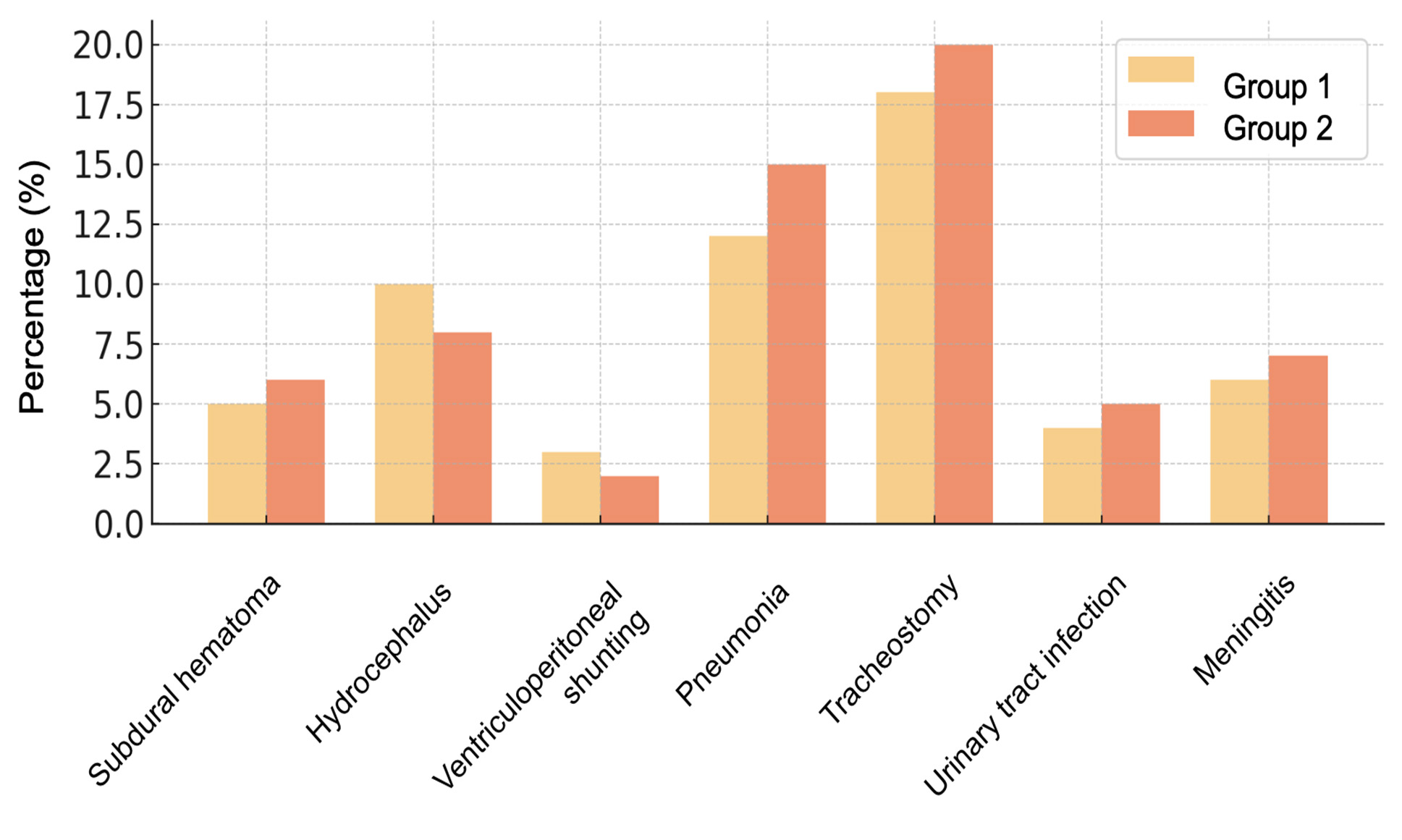
Fig.7. Complications
In Group 1, vasospasm occurred in 36 patients (50.8%), with clinical manifestations in 7 patients (9.9%), angiographically confirmed in 9 patients (12.7%), and delayed cerebral ischemia in 20 patients (28.2%). Mortality in patients with vasospasm was 19.4%, compared to 11.4% in those without vasospasm (Fig. 8).
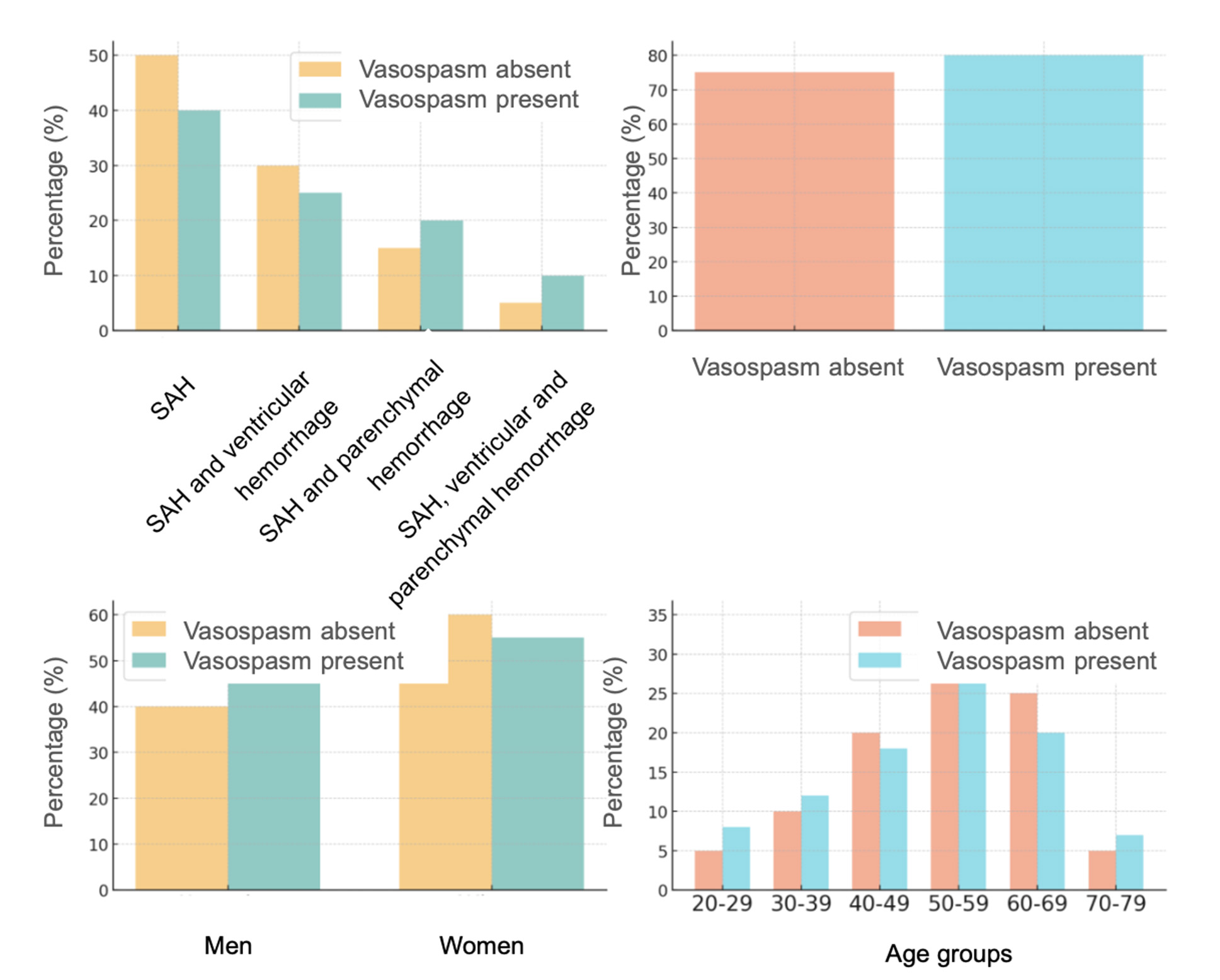
Fig. 8. Vasospasm by age, gender, comorbidities, and hemorrhage type in Group 1
Among women in Group 1, vasospasm was slightly less frequent than in men. Older patients (60–70 years) predominated in the non-vasospasm group, while vasospasm was evenly distributed across different age groups.
Comorbidities were present in 72 patients (78.3%). The proportion of patients with vasospasm and comorbidities was higher, indicating a potential link between comorbidities and an increased risk of vasospasm.
The most common hemorrhage type in both groups was SAH, but complex hemorrhages (e.g., combinations of SAH with ventricular and parenchymal hemorrhage) were more often associated with vasospasm.
In Group 2, comorbidities were observed in 53 patients (74.6%), with vasospasm detected in 11 patients (52.4%), of whom 2 had clinical manifestations (9.5%), 2 were angiographically confirmed (9.5%), and 7 developed delayed cerebral ischemia (33.3%). Mortality among patients with vasospasm was 18.2%, compared to 10.0% in those without vasospasm.
Women with a higher number of MIA aneurysms had a slightly higher frequency of vasospasm compared to men.
In Group 2, significant differences were observed between age groups. Vasospasm occurred more frequently in the 45, 50, 55, and 70-year-old groups. In these age categories, vasospasm was more common, while other age groups demonstrated either a low frequency or absence of vasospasm.
The frequency of vasospasm was significantly higher in individuals with comorbidities compared to those without (Fig. 9).
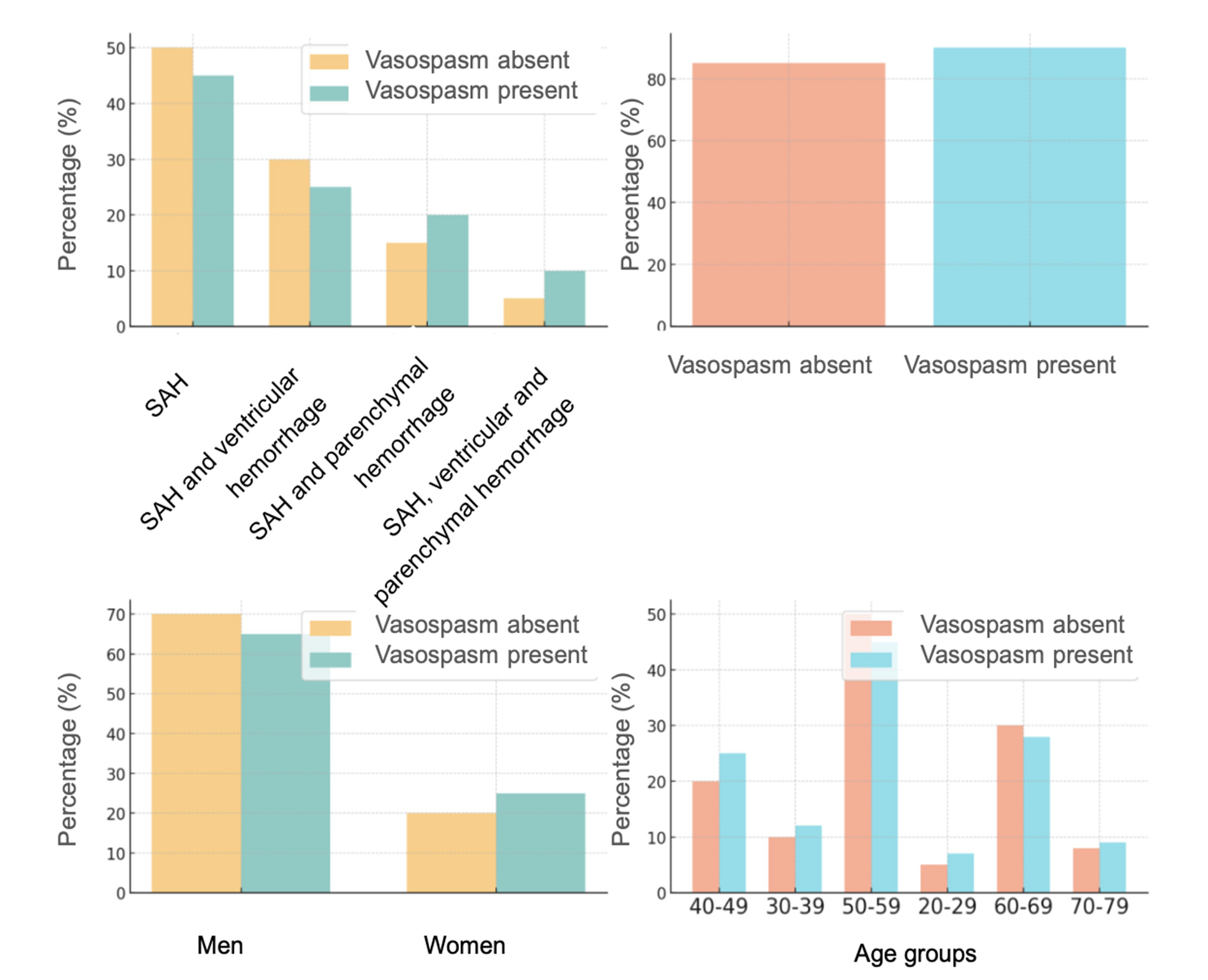
Fig. 9. Vasospasm by age, gender, comorbidities, and hemorrhage type in Group 2
More complex forms of hemorrhage, such as a combination of SAH with ventricular and parenchymal hemorrhages, were more often associated with the development of vasospasm.
An analysis of the presented results using binomial logistic regression revealed several trends and patterns that did not reach statistical significance but overall reflected the specific features of MIA progression. While statistical significance (e.g., p < 0.05) is considered a convenient indicator, the 0.05 threshold is conditional, and the p-value alone is not suitable for guiding clinical or scientific decisions. The p-value indicates (statistical) probability rather than (clinical) certainty, statistically characterizing individual comparisons but without clinical interpretation. A smaller sample size can substantially affect the p-value.
Multifactorial analysis indicates a statistically significant cumulative impact of the analyzed factors (p = 0.023). For example, the presence of comorbidities was 3.4 times more likely among patients with ≥3 aneurysms (OR = 3.4233, 95% CI 0.660–17.762, p = 0.143).
Patients in Group 2 were 1.9 times more likely to be admitted with a WFNS score of 2 (p = 0.335).
A WFNS score of 3 at admission was twice as frequently recorded in Group 1 (p = 0.447). Patients in Groups 1 and 2 were admitted equally often with a WFNS score of 4 (p = 0.978). The likelihood of being admitted with a WFNS score of 5 was 1.8 times higher for patients in Group 2 (p = 0.830) (Fig. 10).
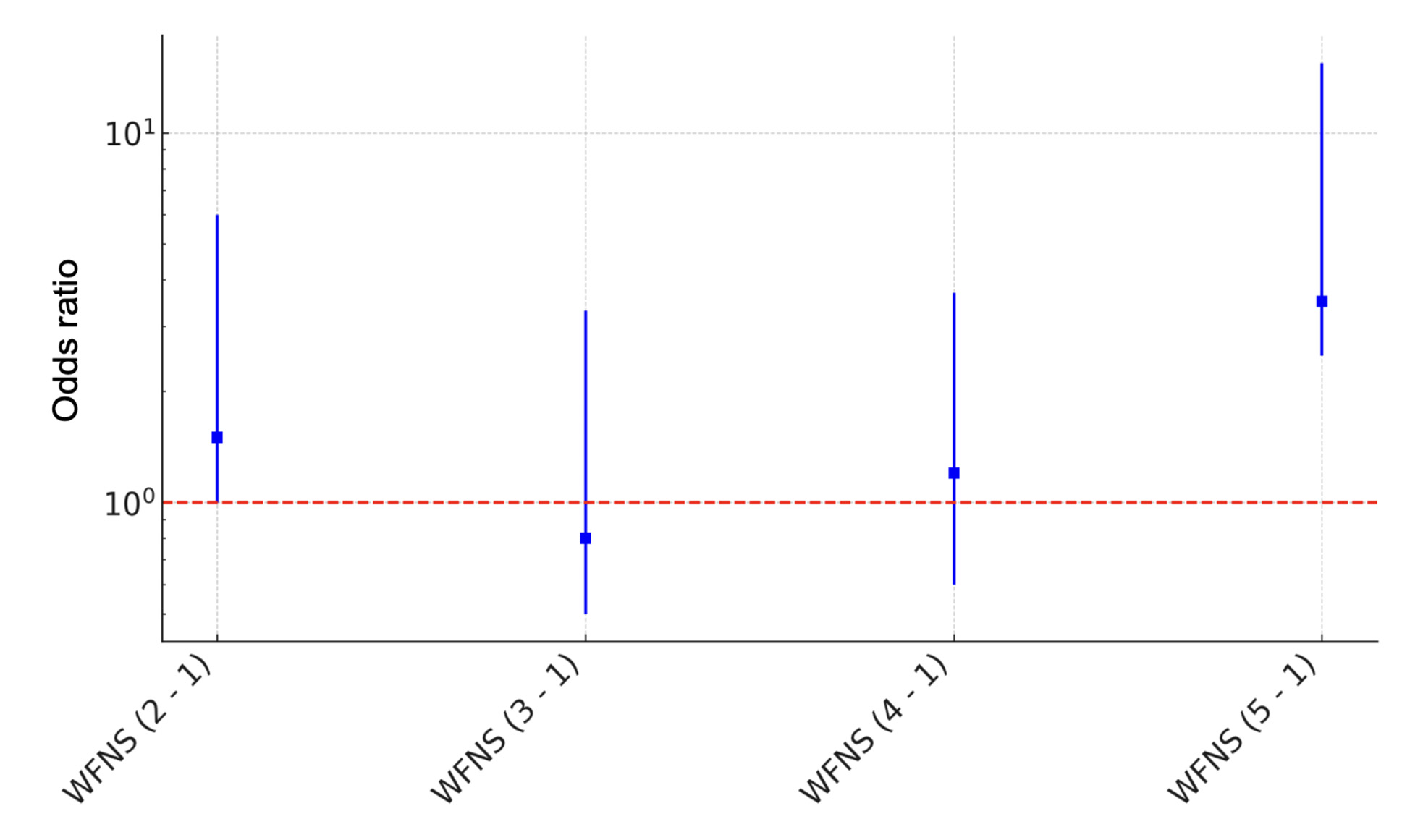
Fig. 10. Logistic regression plot with odds ratios and confidence intervals
The presence of meningeal syndrome increased the risk of having ≥3 aneurysms by more than four times (odds ratio (OR) = 4.41, 95% confidence interval (CI) 0.41–47.13, p = 0.21). The presence of motor impairments significantly reduced the risk of having ≥3 aneurysms (OR = 0.63, 95% CI 0.09–4.18, p = 0.63). Patients in Group 2 had a slightly higher probability of developing vasospasm (OR = 1.22, 95% CI 0.34–4.31, p = 0.752). The presence of comorbidities increased with the number of aneurysms (OR = 3.42, 95% CI 0.65–17.62, p = 0.143).
Conclusions
Comorbidities increased the likelihood of ≥3 aneurysms by over threefold. Patients with fewer aneurysms were twice as likely to present in a milder condition (WFNS score 2). The likelihood of motor deficits decreased 4.4 times with an increased number of aneurysms. Vasospasm risk slightly increased with the number of aneurysms.
The probability of WFNS severity 5 on admission to hospital for group 2 patients was 1.8 times higher than for group 1 patients. The probability of motor deficits decreased 4.4 times with the number of aneurysms. The probability of vasospasm development slightly increased with the number of aneurysms.
Disclosure
Conflict of Interest
The authors declare no conflict of interest.
Ethical Standards
All procedures performed on patients during the study adhered to the ethical standards of the institutional and national ethics committees, as well as the 1964 Helsinki Declaration and its subsequent amendments or comparable ethical standards.
Informed Consent
Written informed consent was obtained from all patients.
Funding
The study was not sponsored.
References
1. Li Y, Bai X, Tu H, Zou Z, Huang Y, Cai J. Multiple intracranial enlarging dissecting aneurysms: a case report. BMC Neurol. 2023 Jul 12;23(1):265. https://doi.org/10.1186/s12883-023-03303-6
2. Deniwar MA. Management of multiple and unruptured cerebral aneurysms. Egypt J Neurosurg. 2022;37:26. https://doi.org/10.1186/s41984-022-00170-0
3. Bakker MK, Ruigrok YM. Genetics of Intracranial Aneurysms. Stroke. 2021 Aug;52(9):3004-3012. https://doi.org/10.1161/STROKEAHA.120.032621
4. Schilling AM, Heidenreich JO, Oldenburg AC, Pietilä T, Stendel R, Wolf KJ. Multiple cerebral aneurysms in factor VII deficiency. AJNR Am J Neuroradiol. 2004 May;25(5):784-6.
5. Wang S, Wang J, Niu Z, Zhang K, Yang T, Hou S, Lin N. Causal relationship between mitochondrial-associated proteins and cerebral aneurysms: a Mendelian randomization study. Front Neurol. 2024 Jul 17;15:1405086. https://doi.org/10.3389/fneur.2024.1405086
6. Grochowski C, Litak J, Kulesza B, et al. Size and location correlations with higher rupture risk of intracranial aneurysms. J Clin Neurosci. 2017; S0967586817314479. doi:10.1016/j.jocn.2017.10.064
7. Sato T, Matsushige T, Chen B, Gembruch O, Dammann P, Jabbarli R, Forsting M, Junker A, Maderwald S, Quick HH, Ladd ME, Sure U, Wrede KH. Correlation Between Thrombus Signal Intensity and Aneurysm Wall Thickness in Partially Thrombosed Intracranial Aneurysms Using 7T Magnetization-Prepared Rapid Acquisition Gradient Echo Magnetic Resonance Imaging. Front Neurol. 2022 Feb 18;13:758126. https://doi.org/10.3389/fneur.2022.758126
8. Jiang H, Weng YX, Zhu Y, Shen J, Pan JW, Zhan RY. Patient and aneurysm characteristics associated with rupture risk of multiple intracranial aneurysms in the anterior circulation system. Acta Neurochir (Wien). 2016 Jul;158(7):1367-75. https://doi.org/10.1007/s00701-016-2826-0