Review article
Ukrainian Neurosurgical Journal. 2024;30(3):3-17
https://doi.org/10.25305/unj.308080
Department of Neurology and Neurosurgery, Ivano-Frankivsk National Medical University, Ivano-Frankivsk, Ukraine
Received: 09 July 2024
Accepted: 14 August 2024
Address for correspondence:
Viacheslav S. Botev, Department of Neurology and Neurosurgery, Ivano-Frankivsk National Medical University, 2 Halytska st., Ivano-Frankivsk, 76018, Ukraine, e-mail: vyacheslav56@yahoo.co.uk
Trigeminal Neuralgia (TN) has been described in the literature as one of the commonest types of craniofacial pain disorders. TN refers to recurrent lancinating pain that occurs in the distribution of one or more branches of the fifth cranial nerve. The pain perception is typically unilateral, abrupt in onset, brief in duration, and usually starts after trivial stimuli.
The overall prevalence of TN was reported around 0.7/1000 persons, but it tends to be higher in more advanced age groups since the initial onset of the symptoms most frequently starts at the age of 50–60 years.
Although TN is more commonly seen in adults, pediatric TN represents <1.5% of all cases. Pediatric TN differs from adult TN primarily being bilateral in nature (42%) and associated with compression of multiple cranial nerves (46%).
This review will evaluate the current surgical procedures used for the treatment of TN. Operative interventions for TN include microvascular decompression (MVD), balloon compression (BC), radiofrequency thermocoagulation (RF TC), glycerol rhizotomy (GR), and stereotactic radiosurgery (SRS). We review the historical development, advantages, and limitations of these operations.
Additionally, we compare specific parameters for all current surgical procedures. We evaluated the short- and long-term outcomes, risk factors, complications and side effects in patients with TN who underwent operations. Arguments for and against the use of surgery for TN are presented.
Next, surgical decision-making algorithm for refractory classical or idiopathic TN is proposed for patients who require surgery. This algorithm may be used by neurosurgeons in selecting the best surgical treatment.
Lastly, we show the data on current clinical trials, the role of genetics to search for genes predisposing to TN. This project begins with the presumption that the risk for developing classical TN is in large part determined genetically. If so, given the power of modern genetic analysis, it should be possible to identify the underlying gene(s).
At present, there is no ideal surgical procedure for trigeminal neuralgia—one that is minimally invasive, uniformly effective, lacking complications, and without failures or recurrences. MVD still remains the standard by which all other contemporary procedures are measured. MVD provides the longest pain-free interval, yet it is not free of morbidity and mortality. Stereotactic radiosurgery provides a reasonable noninvasive option, but it has delayed onset and a recurrence interval (a few years).
Keywords: microvascular decompression; balloon compression; radiofrequency thermocoagulation; glycerol rhizotomy; stereotactic radiosurgery
Trigeminal Neuralgia Awareness Day is observed annually on October 7th.
History and etymology
The renowned medieval Persian scholar, philosopher, and physician Ibn Sina, also known as Avicenna (980–1037), mentioned a condition equivalent to TN in his book “Canon of Medicine”—a pain over the facial skeleton, numbness, and involuntary facial tics. His understanding that nerves conducted pain was later shared by the scholar Esmail Jorjani (1042–1137), a Persian physician. Jorjani described syndromes that were probably consistent with trigeminal neuralgia, hemifacial spasm, and Bell’s palsy in his book “Treasure of the Khawarazm Shah” where he concurred with Avicenna in that pain was conducted via nerves, yet he also implicated an artery-nerve conflict as an etiology of trigeminal neuralgia. [1].
In 1756, Nicholas André introduced the term "tic douloureux," encompassing facial pain and clonic spasms in the face. The name was adopted despite the absence of facial tics in all patients suffering from the disease.
In 1773, John Fothergill published his experience treating 14 patients and identified neuralgia as a manifestation of a certain type of cancer, rather than a convulsive disorder. Due to his detailed clinical descriptions of TN, the disease became known after its author as "Fothergill's disease."
In 1820, Scottish surgeon and anatomist Charles Bell was the first to demonstrate the connection between this syndrome and the trigeminal ganglion, introducing the term "trigeminal neuralgia" into scientific discourse.
Epidemiology
In the United States, 15,000 new cases of trigeminal neuralgia (TN) are registered annually, 1,500 in Canada, and 2,000 in Spain. In the G7 countries (USA, UK, France, Germany, Italy, Spain, and Japan), the number of patients with TN exceeds 1 million, while in China, according to Omics International, there are 4,458,090 cases, which constitutes 2.6% of the population. This figure is likely much higher due to the low rate of seeking medical care. Researchers attribute this primarily to cultural coping strategies for pain.
According to WHO data, the geriatric population is expected to reach 2 billion by 2050, compared to 900 million in 2015. This demographic shift will lead to an increase in the number of potential patients with TN [2].
TN can first appear at any age, but in more than 90% of cases, it begins after the age of 40, with peak incidence between the ages of 37 and 67. The average age of onset for classic TN is 53 years, while patients with secondary TN tend to be about 10 years younger, with an average onset age of 43.
Patients with multiple sclerosis have a 20-fold increased risk of developing TN compared to the general population. Pediatric TN is more often bilateral (42%) compared to adult TN. This is associated with the compression of multiple cranial nerves (46%) due to congenital abnormal vessels, vascular malformations, tumors, cysts, aneurysms, or arachnoiditis [3].
A painful paroxysm is usually followed by a refractory period during which pain cannot be triggered. Usually, attacks occur during the daytime with motor activities involving speech, masticatory and mimic muscles. The course of the disease is chronic and remitting. Preventive measures are lacking [4-6].
Bibliometric Analysis
Publication statistics on trigeminal neuralgia for the years 2001–2021:
4,112 articles,
12,790 authors
Most productive authors:
The highest number of publications comes from the USA (1,205), China (610), and Japan (230) [7].
Anatomy of the trigeminal nerve (TN)
The trigeminal nerve is the largest cranial nerve, providing sensory innervation to the face and motor impulses to the masticatory muscles. Sensory information, including touch, pain, and temperature, is transmitted through the TN nuclei in the pons before reaching the thalamus and, ultimately, the somatosensory cortex.
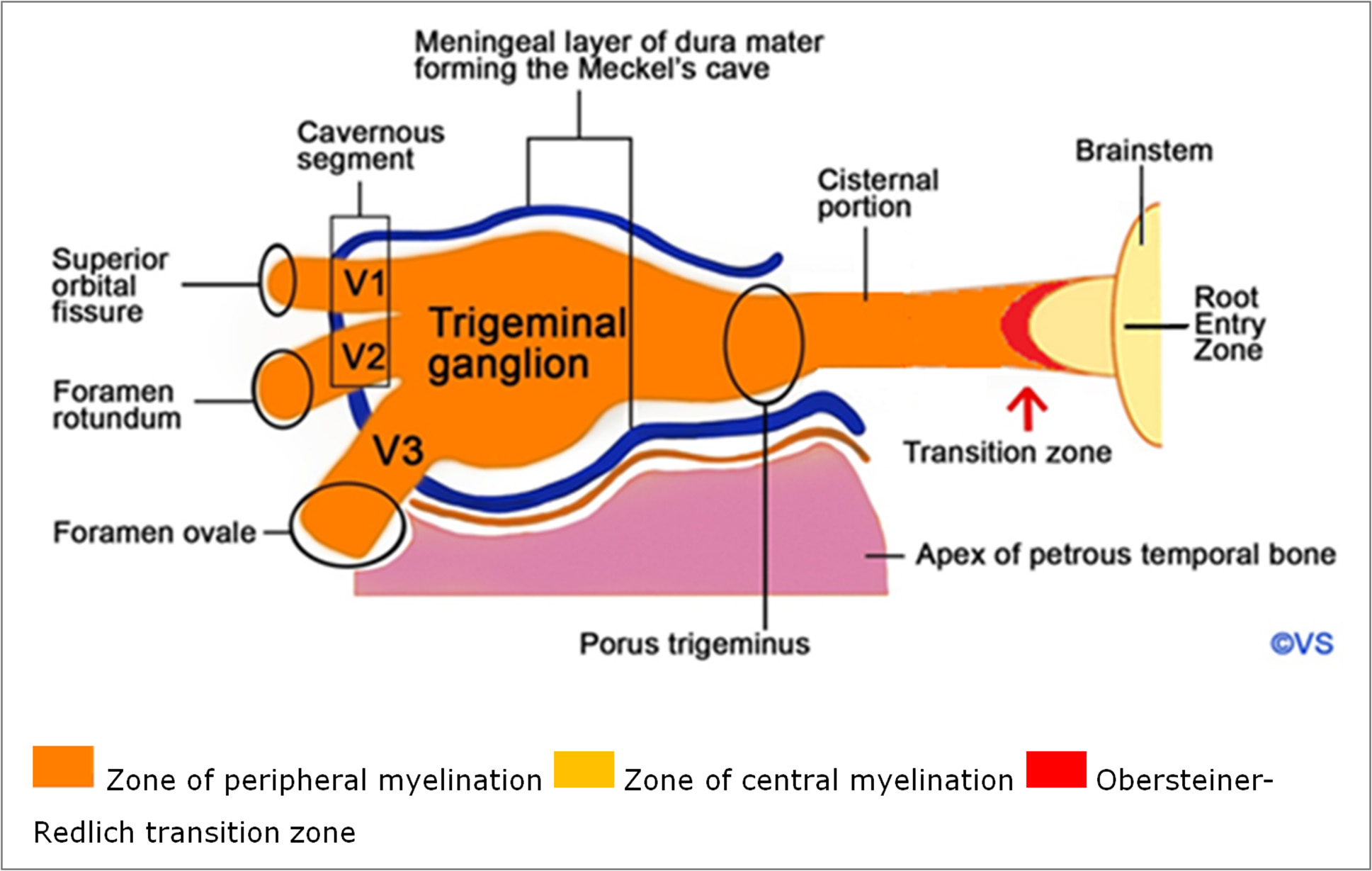
The TN originates from the median anterolateral surface of the pons, with a large sensory and a smaller motor root. It then passes through the prepontine and cerebellopontine cisterns and enters through the dura mater opening (porus trigeminus) into the cerebrospinal fluid-filled space known as Meckel's cave, which contains the relatively large trigeminal ganglion (Gasserian ganglion), measuring 15–18 mm. Inside Meckel's cave, the trigeminal ganglion divides into three branches: ophthalmic (V1), maxillary (V2), and mandibular (V3) (Fig. 1).

Fig. 1. Trigeminal nerve anatomy: V1- ophthalmic nerve, V2 – maxillary nerve, V3 - mandibular nerve
The nerve consists of five segments: the brainstem, cisternal segment, Meckel's cave, cavernous and peripheral segments. The cisternal segment is almost always the source of classic TN caused by vascular compression, identified by specific radiological criteria:
- The corresponding blood vessel is almost always an artery.
- The nerve and blood vessel cross perpendicularly.
- The contact between blood vessel and nerve occurs in the root entry zone of the nerve.
TN compression most frequently occurs due to contact with the superior cerebellar artery, less often with the anterior inferior cerebellar artery or smaller branches of the basilar artery. Only a few cases have been reported where the compression was caused by contact with an aneurysm, vertebrobasilar dolichoectasia, AVM, AV fistula, or vein.
The Redlich–Obersteiner zone, located just at the pons, contains a glial cone of residual central myelination that passes into the peripheral myelination of the nerve through Schwann cells. The junction of the central and peripheral nervous systems is particularly sensitive to mechanical stress.
Pharmacotherapy
First-line treatment includes monotherapy with sodium channel blockers. This group of drugs includes carbamazepine and oxcarbazepine. Carbamazepine is effective in more than 60% of patients, but 30% experience adverse reactions, that are severe occurring in one out of every 24 patients receiving treatment. As for oxcarbazepine, it was discontinued in Phase IV trials according to recent reports on the DrugBank website [8].
In October 2022, the FDA granted priority status (Fast Track) to a new drug under development called "Basimglurant" (NOE-101). Basimglurant is a potent inhibitor of the metabotropic glutamate receptor 5 (mGluR5), which is overproduced in cases of TN [9].
The average active period of TN lasts 49 days, followed by remission for several months (36%), weeks (16%), or days (16%). Only 6% of patients can expect remission lasting more than a year, and about 20% may suffer from continuous attacks [10].
When a patient experiences a complete absence of pain for a sufficiently long period (about 6 months, as per GECSEN recommendation), drug withdrawal may be considered. In any case, the discontinuation of treatment should be gradual. Surgical methods are recommended if pharmacotherapy is ineffective, poorly tolerated, or if its effectiveness decreases over time.
The optimal time for surgery is not definitively established, though it is reasonable to avoid excessive delays. Surgery should be offered after the first year of no improvement or intolerability to pharmacotherapy. Overall, significant therapeutic outcomes should not be expected if drugs from three different classes, with different mechanisms of action, have already been tried (alone or in combination), in appropriate doses, and for a maximum period of 3 months per drug, to establish their ineffectiveness.
Surgical and other treatment methods for TN
First and foremost, it is important to note that the term "destructive" is more appropriate than "ablative." This is because "ablation" refers to the removal of tissue from its original location, but not necessarily its destruction (English dictionaries Collins, Cambridge, Macmillan English Dictionary. Ablation – the surgical removal of an organ or part).
Surgical options for treating trigeminal neuralgia include microvascular decompression and a range of destructive procedures such as percutaneous balloon compression, glycerol rhizotomy, radiofrequency thermorhizotomy, or stereotactic radiosurgery targeting the Gasserian ganglion (Table 1).
Тable 1. Summary of available treatment modalities for trigeminal neuralgia [11, with modifications]
|
Surgery |
Destructive |
Stereotactic radiosurgery |
Gamma-Knife, |
|
Percutaneous rhizotomy techniques |
Glycerol injection, |
||
|
Partial sensory neurectomy |
|||
|
Non-destructive |
Microvascular decompression |
||
|
Neuromodulation |
Peripheral techniques |
Trigeminal branch stimulation, |
|
|
Central techniques |
Motor cortex stimulation, |
||
|
Subdermal therapies |
Botulinum toxin type A, |
||
|
Other therapies |
Cryotherapy, |
||
Since the end of the last century, many renowned surgeons have been involved in the treatment of TN.
The key stages of the development of surgical methods are presented in (Table 2).
Тable 2. History of surgery for trigeminal neuralgia [12, with modifications]
|
Year |
Authors |
Procedures |
|
1892 |
Hartley F., Krause F. |
Excision of the Gasserian ganglion and its roots |
|
1900 |
Cushing HW |
Trigeminal ganglionectomy |
|
1901 |
Spiller WG, Frazier CH |
Extradural sub-temporal retrogasserian neurotomy |
|
1911 |
Taptas JN |
Alcohol injection into Meckel’s cavity |
|
1914 |
Härtel F. |
Percutaneous approach to the foramen ovale |
|
1932 |
Kirschner M. |
Percutaneous electrocoagulation of the trigeminal ganglion |
|
1932 |
Dandy WE |
Posterior fossa subtotal rhizotomy |
|
1934 |
Dandy WE |
Recognition of vascular compression as a cause of neuralgia |
|
1947 |
Olivecrona AH |
Subtemporal rhizotomy |
|
1951 |
Taarnhøj P. |
Intradural decompression of the trigeminal ganglion and the posterior root |
|
1952 |
Love JG |
Extradural decompression of the trigeminal ganglion and the posterior root |
|
1955 |
Shelden CH |
Enlarging the foramen ovale and foramen rotundum |
|
1959 |
Gardner WJ, Miklos MV |
First report of vascular decompression |
|
1966 |
Jannetta PJ, Rand RW |
Transtentorial retrogasserian microvascular decompression |
|
1971 |
Jannetta PJ |
Retromastoid microvascular decompression |
|
1971 |
Leksell L. |
Stereotactic Gamma-Knife Radiosurgery |
|
1974 |
Sweet WH, Wepsic JG |
Radiofrequency thermocoagulation |
|
1981 |
Håkanson S, Sweet WH |
Percutaneous glycerol injection into Meckel’s cavity |
|
1983 |
Mullan S., Lichtor T. |
Percutaneous balloon compression |
|
1993 |
Meyerson BA, Lindblom U. |
Motor cortex stimulation |
Percutaneous destructive methods
In 1914, Fritz Härtel performed the first anterior percutaneous puncture of Meckel's cave using a spinal needle placed anterior to the coronoid process of the mandible through the foramen ovale; this approach is still in use today (Fig. 2) [13].
 A
A 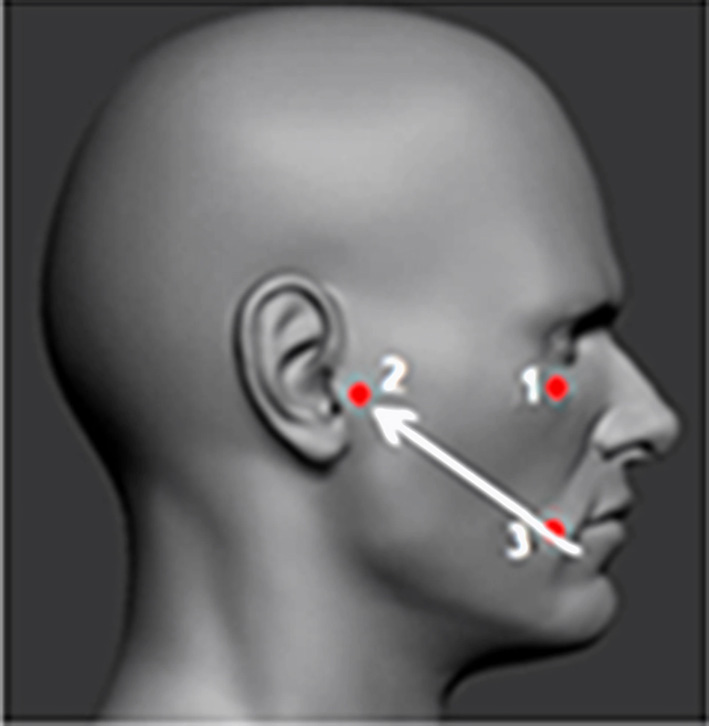 B
B 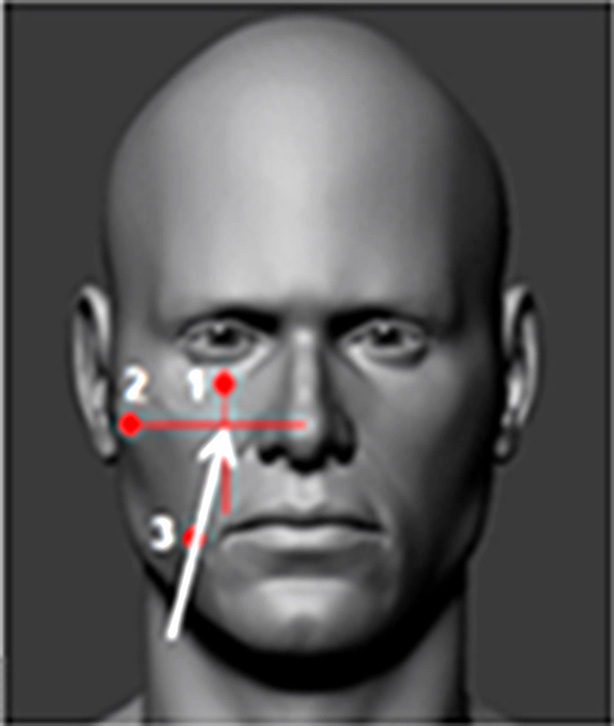
Fig. 2. Fritz Härtel (1877–1940). Härtel’s approach: A – lateral view, B - anteroposterior view
The needle/cannula/trocar is inserted 2.5–3 cm lateral to the corner of the mouth (3). The needle’s trajectory is directed towards the intersection of two planes: one sagittal plane passing through the ipsilateral pupil (1) and the other coronal plane located 3 cm anterior to the external auditory canal (2). This point approximately corresponds to the lateral projection of the foramen ovale on the skin.
The inverted pyramid is another simple yet effective model for guiding the needle to the foramen ovale. Three of the four vertices of the pyramid are cutaneous and visible to the surgeon, while the fourth is the foramen ovale, which requires spatial imagination.
The foramen ovale is located at the posterior aspect of the greater wing of the sphenoid bone. It transmits the mandibular nerve, the accessory meningeal artery, the lesser superficial petrosal nerve, and the emissary vein. This foramen is one of the key points situated at the transition zone between intracranial and extracranial structures.
A thorough understanding of surgical anatomy and careful preoperative imaging are crucial for recognizing the potential risks of Härtel’s percutaneous approach. A misaligned trajectory may lead to complications, particularly puncturing the internal carotid artery. Even with correct trajectory, the needle may penetrate the parotid duct, maxillary artery, or Eustachian tube, leading to complications such as hemoptysis, hematoma of the cheek and/or pterygoid-mandibular area, serous otitis, and others (Fig. 3).
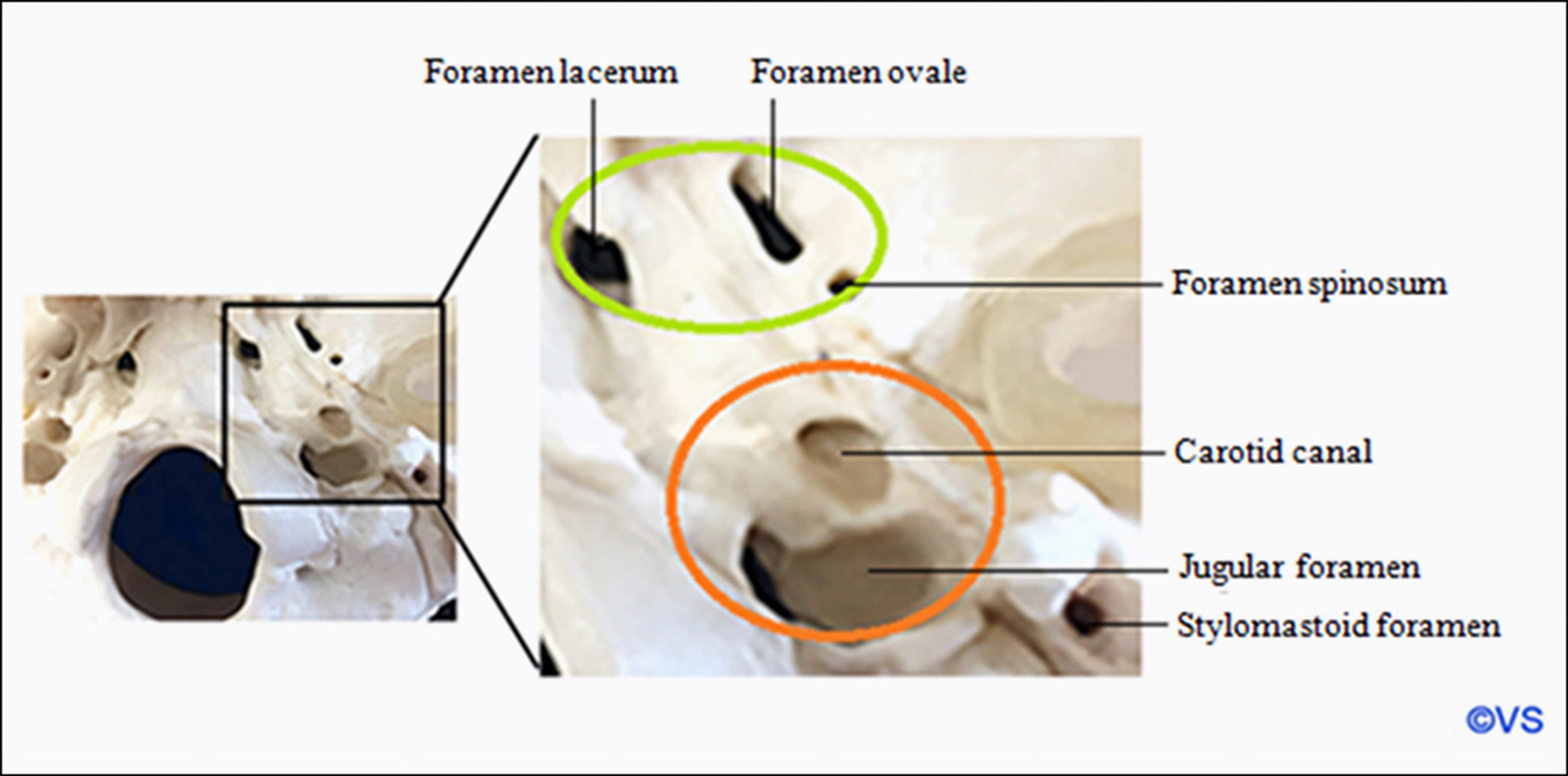
Fig. 3. The safe zones and danger zones along the periforaminal space. The green zone defines the safe zones to “walk” the needle along the skull base to find the foramen. The orange zone defines danger zones where the carotid artery or jugular vein could be injured
Accessing and puncturing the foramen ovale can be challenging in certain patients, particularly those with hypertrophic pterygoid processes or platybasia. In such cases, a trajectory guided by neuronavigation systems, or even better, catheterization of the foramen under CT guidance, may be helpful.
Thermocoagulation of the Gasserian ganglion / Radiofrequency (RF) destruction
The procedure is referred to as radiofrequency because it uses the same frequency as AM radio—approximately 500 kHz of electromagnetic radiation. Electrocoagulation for the treatment of the trigeminal nerve roots was first developed by A. Réthi in 1913. This method was associated with severe complications, including corneal ulcers that required enucleation, multiple cranial nerve palsies, carotid artery damage, cardiac arrest, meningitis, and death.
Further development of the method was continued by Martin Kirschner, who in 1931 developed a stereotactic method for inserting an insulated needle through the foramen ovale for electrocoagulation of the Gasserian ganglion. Kirschner reported on 250 cases in 1936 and 113 cases in 1942 [14, 15].
William H. Sweet (Fig. 4) and James G. Wepsic further developed the technique of radiofrequency thermal destruction of the trigeminal nerve root in 1974 [16]. They introduced various control measures—electrical stimulation of the root and temperature monitoring. In 1982, H. van Loveren, John M. Tew, and J.T. Keller presented an electrode with a curved tip (Fig. 5) [17].

Fig. 4. William Herbert Sweet (1910–2001)
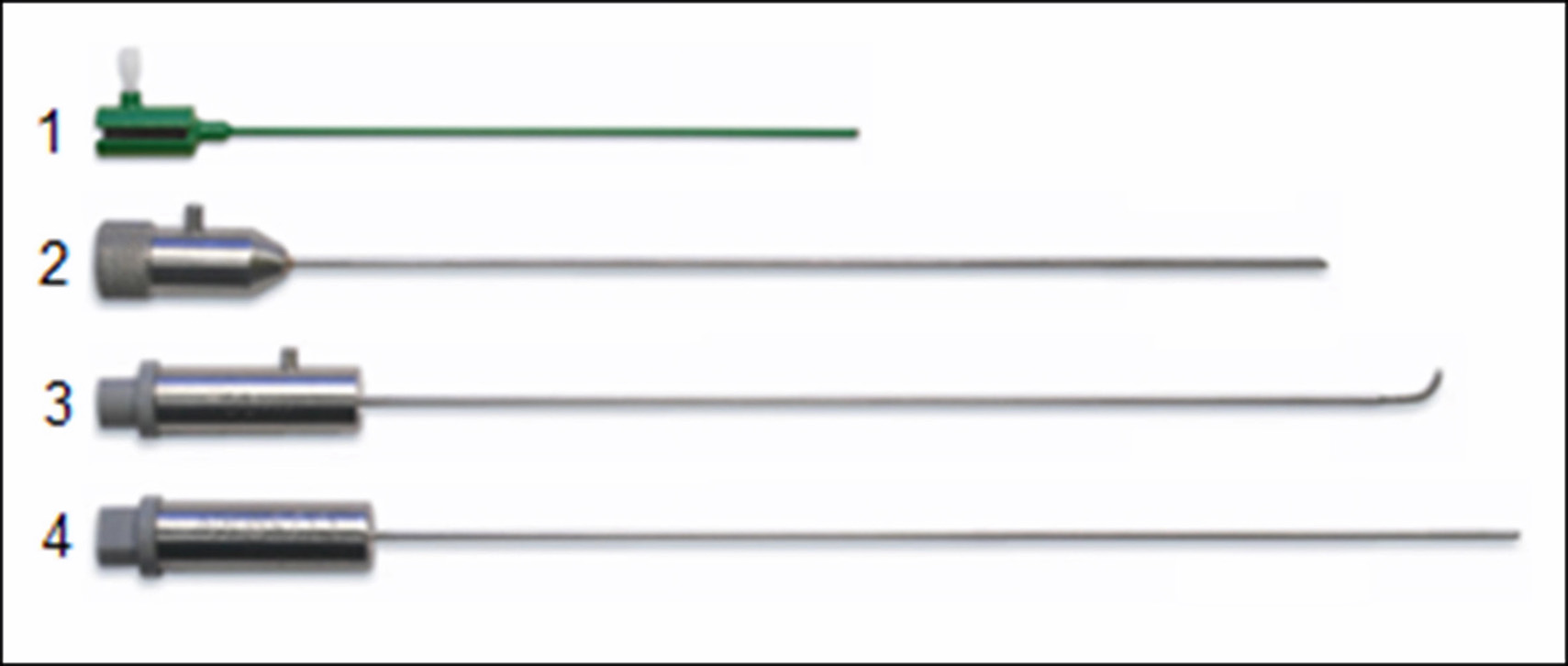
Fig. 5. Radiofrequency thermocoagulation kit: 1 - cannula, 2 - stylet, 3 - curved electrode, 4 - straight electrode
Straight electrodes are preferred for third branch neuralgia, and curved electrodes for first or second branch neuralgia.
Continuous RF destruction is applied for 60-90 seconds, temperature 70-90°C. During pulsed RF destruction, intervals of short bursts of current lasting 20 ms alternate with pauses lasting 480 ms. Temperature does not exceed 42°C.
Yücel Kanpolat et al. (2001) described their 25-year experience in treating 1600 patients (2138 operations). 1216 patients underwent a single operation and 384 underwent multiple operations: twice in 275 patients, three times in 79, four times in 19, five times in 8 and six times in 3. Initial analgesic effect was noted in 97.6% of patients. Early recurrence of pain (< 6 months) was observed in 123 patients and late recurrence (> 6 months) in 278 patients. The recurrence rate was 25.1% during a mean follow-up period of 68 months. After 5 years, complete analgesia was maintained in 58% of patients who underwent a single procedure; this number decreased to 52% after 10 years and to 41% after 20 years. The most important complications were hypoesthesia with paresthesias in 14.6% and masticatory muscle dysfunction in 4.5% of cases. Absence or reduction of corneal reflex was observed in 91 patients (5.7%). Among them, 10 developed keratitis [18].
Review by M. Sindou and M. Tatli (2009) conducted by the French Neurosurgical Society (10 articles, 7483 patients) with a follow-up period of 3 to 26 years showed a mean incidence of an initial analgesic effect of 94% (81 to 99% depending on the series) and a mean long-term efficacy rate of 60.4% (20 to 93%). Side effects and complications were reported with varying frequency: bothersome facial hypoesthesia 5-98%, difficulty in mastication 4-24%, keratitis 1-8% and dysesthesia/anesthesia dolorosa 0.8-7% for later complications. Mortality was 0.1% due to carotid artery damage [19].
Analysis by L. Bendtsen et al. performed by the European Academy of Neurology 2019 (7 articles, 4533 patients) with a follow-up period of 3 to 9.3 years reported an initial analgesic effect of 26 to 82% of cases and a recurrence rate ranging from 16 to 74%. There were no fatal outcomes in this review. Patients with hearing loss were 6, cranial nerve palsy 36, corneal hypoesthesia 300, keratitis 55, masticatory weakness 280, facial hypoesthesia 853, anesthesia dolorosa 29 [20].
Percutaneous balloon compression of Gasserian ganglion
In 1983, Sean Mullan (Fig. 6) and Terry Lichtor performed percutaneous balloon compression (BC) of the Gasserian ganglion [21]. Mullan's kit contains a size 14 hollow metal stylet (introducer), sharp and blunt obturators, curved and straight probes, and No.4 Fogarty balloon catheter. The balloon is inflated to a pressure of 1.0-1.5 atmospheres for 60-90 seconds. Ideally, a pear-shaped configuration of the balloon in the porus trigeminus is created during compression (Fig. 7) [22]. Some redness of the ipsilateral conjunctiva is often observed after compression.

Fig. 6. John Francis Mullan (1925–2015)
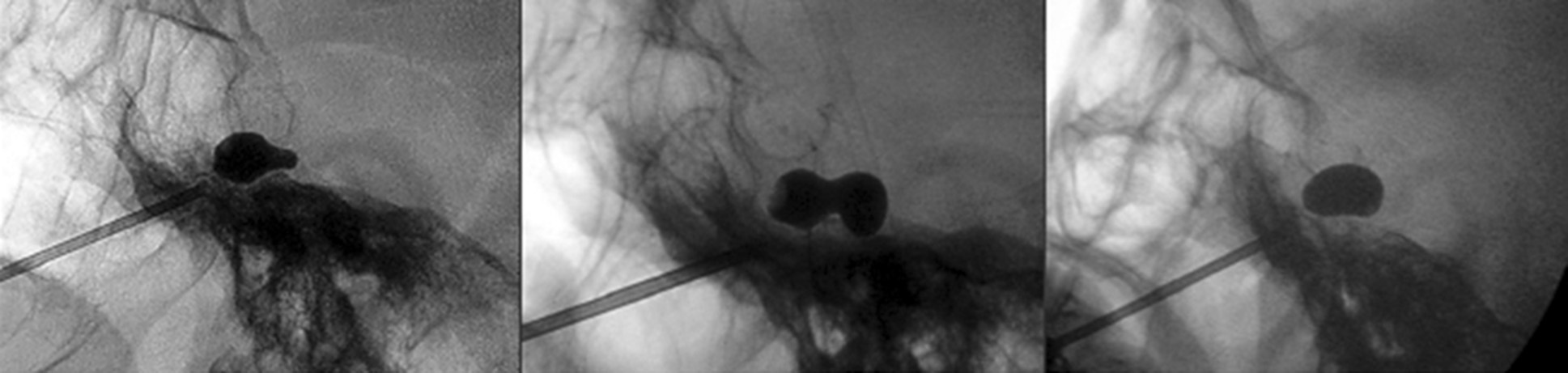
Fig. 7. Balloon shape: A - pear, B - hourglass, C - oval [22]
In 2016, the FDA banned the release of Mullan kit due to manufacturing difficulties, so some surgeons use other kits or a standard liver biopsy needle, which is available in most operating rooms (Fig.8) [23-25].
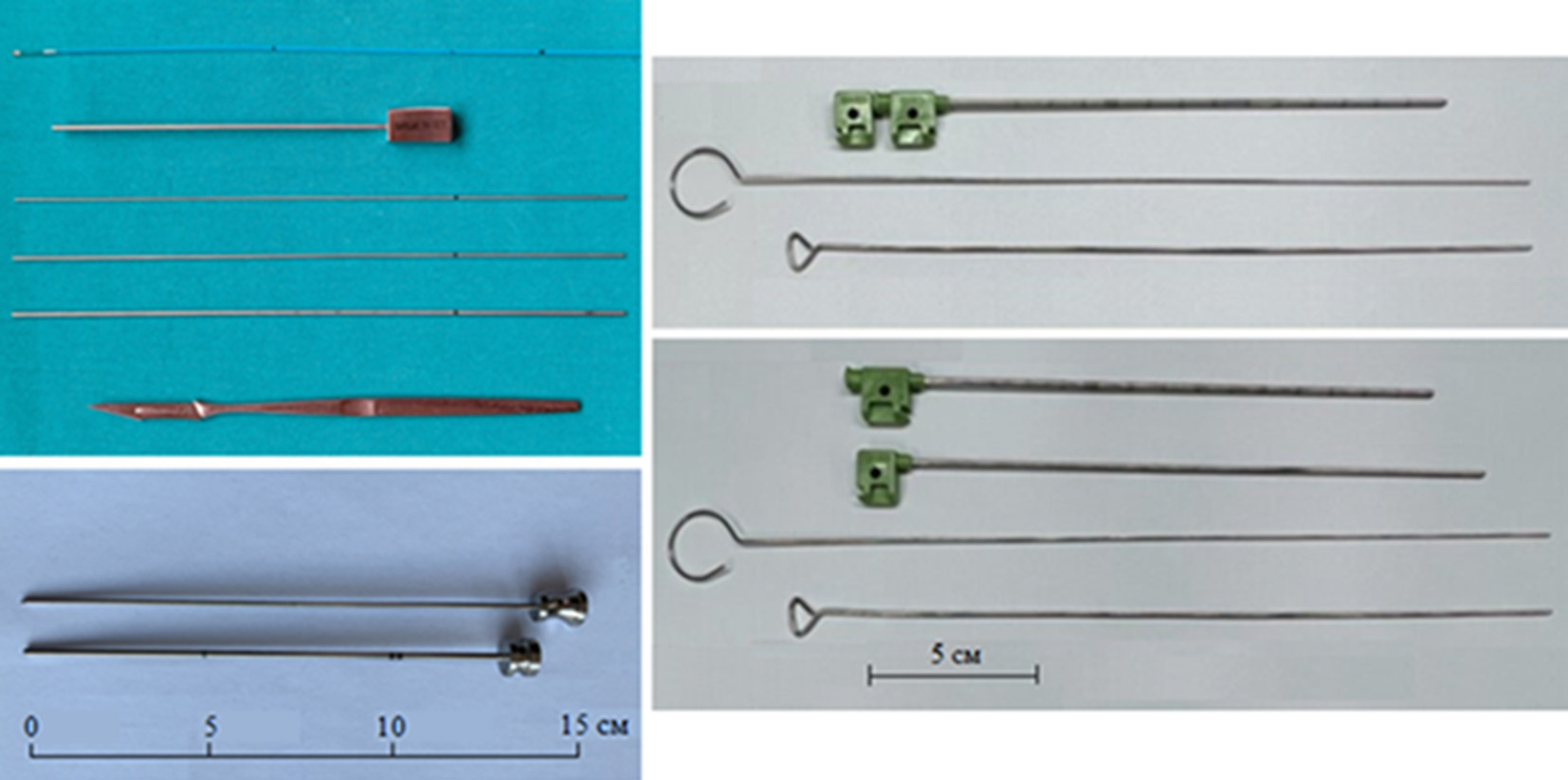
Fig. 8. Various balloon compression (BC) kits: A - O. Barlas [23], B - Y. Ma [24], C - C. Sun [25]
In the review by A. Donnet et al. (2017), conducted by the French Neurosurgical Society (10 series, 1404 patients) with a follow-up period of 1–6 years, the initial pain relief effect was observed in 82–100% of patients (an average of 96%), with long-term efficacy in 54.5–91.3% of patients (an average of 67%). Hypesthesia was reported in 4–77% of cases, and transient masticatory muscle paresis occurred in 50–66% of cases. Mortality rate due to carotid artery damage was 0.2% [19].
S. Grewal et al. (2018) from the Mayo Clinic, in a series of 222 patients followed for 15 years with an average follow-up of 31 months, focused particularly on the timing of recurrences after BC. The likelihood of being pain-free 15 years after the procedure was only 10%. Recurrences occurred on average 12 months post-procedure. A significant number of patients reported some degree of hypesthesia (82%), though in most cases it was relatively mild. At the last follow-up, 88% of patients were pain-free, with a median follow-up of 31.1 months. Atypical pain was associated with poorer outcomes. Repeat procedures carried a higher risk of pain recurrence, but the initial effectiveness of repeat procedures did not diminish [26].
According to a review by L. Bendtsen et al. from the European Academy of Neurology in 2019 (5 papers, 755 patients) with a follow-up period of 5 to 10.7 years, the initial pain relief rate was 95%, which then declined to 54.5–80% (an average of 67%) at the final follow-up. Recurrence or failure rates ranged from 20 to 51.7%. The most significant reported complications were hypesthesia with paresthesia in 14.6% of cases and motor weakness of the trigeminal nerve in 4.5% [20].
Yi Ma, following his training in Rome under Dr. Mario Meglio from 2000 to 2017, performed 12,838 BC procedures on 12,797 patients. The initial pain relief effect was reported in 95.6% of cases. Hemorrhagic complications occurred in 11 patients, with two resulting in death. One patient died from multiple intracerebral hematomas, while the second died of a large subdural hematoma due to cavernous sinus injury. Four patients required ventriculoperitoneal shunting for hydrocephalus after subarachnoid hemorrhage, while the other five recovered spontaneously without surgery.
Five vascular complications occurred, including three cases of dural carotid-cavernous fistula (CCF) and two cases of external carotid artery system fistula. These patients were successfully treated with endovascular embolization. Ischemic stroke occurred in two cases, though the causes were not determined. Kaplan-Meier curves showed a long-term pain relief effect of over 86% five years after BC, over 83% after 10 years, and 77% after 15 years. The annual risk of recurrence was less than 7% after five years, less than 4% after 10 years, and remained stable at 2% from the 15th year post-procedure [24].
A. Kourilsky et al. (2022) performed a retrospective analysis of 131 patients who underwent BC for the first time between 1985 and 2019 in two French hospitals. Potential clinical and radiological predictors of time to pain recurrence and severe sensory complications were assessed using the Cox model and logistic regression, respectively. The median follow-up period was three years. Pain recurrence occurred in 77 patients (58.7%), with a median time to recurrence of two years.
Multivariate analysis identified six independent factors predicting pain recurrence: 1) longer preoperative symptom duration; 2) pain location along the mandibular branch of the trigeminal nerve (V3); 3) atypical pain; 4) a history of multiple sclerosis; 5) the use of a medical device not specifically designed for BC; and 6) balloon compression duration exceeding 60 seconds [27].
Percutaneous retrogasserian glycerol rhizotomy
From 1974 to 1981, Sten Håkanson (Fig. 9) performed percutaneous puncture destruction of the Gasserian ganglion through the foramen ovale using pure glycerol.

Fig. 9. Sten Håkanson
Glycerol (C3H8O3) is a trivalent alcohol that is colorless and odorless, serving as a precursor for the synthesis of phospholipids and triacylglycerols in the liver and adipose tissue of humans. The three hydroxyl groups and carbon backbone of glycerol form a molecule with a universal solubility profile, allowing it to dissolve easily in water and penetrate cell membranes. The mechanism of glycerol neurolysis is due to the disruption of tight junctions between Schwann cells and the external axolemma of peripheral nerves, as well as alterations in intracellular osmolality. It is also believed that anhydrous glycerol isolates the pathological section of the axon, and since it has a high dielectric constant (45.5 at 25°C), it renders the nerve a poor conductor. This causes presynaptic inhibition and delays the propagation of nerve impulses.
In the 1970s in Stockholm, during the development of stereotactic radiosurgical destruction of the Gasserian ganglion using a Gamma-Knife, radioactive tantalum powder dissolved in glycerol was used to create a stereotactic target by injecting it into the trigeminal cistern. Håkanson and his colleagues noticed that the injection of this medium itself seemed to reduce facial pain even before the Gamma-Knife procedure was administered. The group later described a method of direct glycerol injection into the trigeminal cistern and published their first case series in 1981 [28].
In a report by P. Asplund et al. (2016) from Umeå University (Sweden), involving 124 patients who underwent glycerol rhizotomy and were followed up for 5 years, an immediate effect was noted in 85% of cases, with a median pain-free period lasting 21 months. At the last follow-up, 47 patients (38%) were pain-free. Hypesthesia with dysesthesia was observed in 23% of patients, frequently combined with a decreased corneal reflex. Herpes outbreaks occurred in 3%, and chemical meningitis also in 3%. According to Kaplan–Meier statistical analysis, the probability of being pain-free at 5 years was less than 20% [29].
A literature review by the French Neurosurgical Society (2017), including 1310 cases with a follow-up period of 1–10 years (average 6.5 years), showed an effectiveness rate of 42–84%, which is maintained in the long term in 18–59% of cases (average 38.5%). The main complications include: hypesthesia with dysesthesia in 30% of cases, refractory keratitis in 5%, and herpes outbreaks in 50% [19].
In a series by L. Bendtsen et al. (2019), including 289 patients with a follow-up period of 4.5 to 8 years, an immediate effect was achieved in 75% of patients. Pain relief at the average follow-up period decreased to 18–59% depending on the series (average 40%), with the frequency of recurrences or failures varying from 41 to 84%. The most significant complication in these series was facial hypesthesia with varying degrees of paresthesia/dysesthesia in 39% [20].
Imran Noorani et al. (2016) in a series of 152 glycerol rhizotomies with long-term follow-up showed that analgesia of class I or II was noted in 53.7% of procedures, class III in 17.4%, class IV in 16.1%, and class V in 12.8%. Complete initial pain relief (with or without medication) was achieved in 72%, comparable to the 73–98% reported in previous literature. The overall complication rate was 30.3%. The most common complications were decreased corneal reflex (11.2%), recurrence of herpes simplex (8.6%), numbness (71.7%), and mild dysesthesia (5.9%). The average time to pain recurrence was 14 months. The recurrence rate at 12 months was 45.5% [30].
The 5-point Barrow Neurological Institute Pain Scale (BNI-PS) allows for the assessment of pain intensity and the need for the patient to use medications to manage it (Table 3).
Тable 3. Barrow Neurological Institute (BNI) Pain Intensity Scale
|
Score |
Description |
|
I |
No pain, no medication |
|
II |
Occasional pain, not requiring medication |
|
III |
Some pain, adequately controlled with medication |
|
IV |
Some pain, not adequately controlled with medication |
|
V |
Severe pain, no pain relief |
Glycerol rhizotomy has an advantage compared to other methods as it does not require specialized equipment. However, the neurotoxic effects of glycerol, due to the difficulty in controlling its diffusion into the subarachnoid space, lead to side effects and complications.
Comparison of three percutaneous rhizotomy methods
Imran Noorani et al. (2021) were the first to analyze data over a 19-year period at a single neurosurgical center, involving 210 patients (a total of 392 procedures): 152 glycerol rhizotomies, 155 thermocoagulations, and 85 balloon compressions.
It was shown that balloon compression provides the longest duration of pain relief compared to glycerol rhizotomy and thermocoagulation, with the latter two showing similar durations of pain relief. It was also noted that repeated procedures offer good long-term pain relief. Recurrence of pain occurred in 25% of patients within 5 months after glycerol rhizotomy, 12 months after thermocoagulation, and 26 months after balloon compression. The average time to pain recurrence was 29 months for glycerol rhizotomy and 30 months for thermocoagulation [31].
Comparison of balloon compression (BC) with radiofrequency (RF) thermocoagulation
J. Herta et al. (2023) conducted a retrospective single-center analysis of a series involving 230 patients with TN in Austria, who underwent 202 BCs and 234 thermocoagulations between 2002 and 2019. An immediate effect was achieved after 353 (84.2%) procedures, with no significant difference between BC (83.7%) and thermocoagulation (84.9%). The pain-free period following the procedures was longer for BC at 481 days, compared to thermocoagulation at 421 days, but without statistical significance. The complication rate was 22.2%, and zero mortality showed no differences between the two procedures.
Seventeen (3.9%) operations had to be terminated due to severe bleeding (1.6%), inability to access the foramen ovale (1.4%), and anesthetic issues related to airway or circulatory function (0.9%). Diplopia occurred after four BC procedures, but never after thermocoagulation. Bradycardia, which is common with destructive procedures, had to be treated with medication in 60% of all cases. A drawback of both methods is the significant recurrence rate, which varies widely across studies, ranging from 15% to 64%. After one year, the recurrence rate was 56.1% for BC and 56% for RF. In the long term, BC showed a slightly longer recurrence-free period than
thermocoagulation [32].
Comparison of percutaneous rhizotomy methods (Table 4).
Table 4. Comparison of the percutaneous procedures [33]
|
|
RFT |
GR |
PBC |
|
Initial outcome |
97 - 99% |
53 – 98% |
82 – 93% |
|
Recurrence |
+ |
+++ |
++ |
|
Division selectivity |
+++ |
++ |
+ |
|
Nerve fiber selectivity |
++ |
++ |
+++ |
|
Anesthesia |
Awake |
Sedation |
Sedation+pacemaker |
|
Overall complication rate |
+++ |
++ |
+ |
|
Anesthesia dolorosa |
0.6 – 0.8% |
0 – 5% |
0 – 3.4% |
|
Masseter weakness |
3 – 29% |
0 – 4.1% |
10 – 50% |
|
+++: more likely, ++: moderate, +: less likely, RFT: radiofrequency thermocoagulation, GR: glycerol rhizotomy, PBC: percutaneous balloon compression |
|||
Microvascular decompression (MVD)
In 1966, Robert W. Rand (Fig. 10) was the first to perform microvascular decompression (MVD) of the trigeminal nerve root at the brainstem via a subtemporal approach. In 1967, Peter J. Jannetta (Fig. 11) performed MVD of the trigeminal nerve root in the prepontine cistern.

Fig. 10. Robert W. Rand (1923–2013)

Fig. 11. Peter J. Jannetta (1932–2016)
Reproduced with the permission of prof. M. McLaughlin
A large sample of 1,185 cases from 1972 to 1991, in the study by F. Barker, P. Jannetta et al. (1996), demonstrated sustained pain relief in 70% of cases, with 30% experiencing recurrences. 11% underwent repeat surgeries. Two female patients died due to hemispheric stroke and infarction of the brainstem and cerebellum. Six patients developed infarction, edema, or hemorrhage in the ipsilateral cerebellar hemisphere, five of whom underwent cerebellar resection. Two patients were found to have postoperative supratentorial hematomas (one subdural and one intracerebral). These eight patients recovered after hematoma evacuation. After the introduction of intraoperative monitoring of brainstem evoked potentials, there were no fatal complications. Female gender, symptom duration longer than eight years, venous compression, and the absence of immediate postoperative pain cessation were significant predictors of possible recurrence [34].
In 1999, Peter Jannetta's team published a report on 4,415 operations performed between 1969 and 1999. The authors described all the nuances of each of the six main stages of MVD in detail [35].
M. Sindou et al. (2018), in a meta-analysis of 17 articles (5,124 patients), showed similar results across various published articles. Immediate pain relief was achieved in 80–98% of patients (an average of 91.8%), with complete pain relief maintained in 62–89% (an average of 76.6%) by the end of the follow-up period (ranging from 5 to 11 years, with an average of 7 years). Complete pain relief after the first procedure was noted in 71% of cases. Improvement was observed in 93% patients immediately after the intervention [36].
In a meta-analysis by L. Bendtsen et al. (2019) from the European Academy of Neurology (21 articles, 5,149 patients) with an average follow-up period of 3 to 10.9 years, initial pain relief was reported in 80-98.2% of cases, pain-free status during follow-up in 62-89%, and recurrence rates ranging from 4-38% [20].
Complications after MVD may occur in 20% of patients, though serious complications are rare. Complications related to cranial nerves require special attention, as cranial nerves IV through XII are exposed during surgical access. Numbness and dysesthesia occur in 5% to 10% of patients. Diplopia due to manipulation of the fourth or sixth nerve is often transient, and facial nerve paralysis is rare (<1%). Hearing loss varies from 1% to 20%, depending on audiometry or subjective reports.
Other complications include cerebrospinal fluid leakage (3–4%); infections are rare and occur at the same rate as with other craniotomies. Other rare complications include aseptic meningitis, postoperative bleeding, and stroke. The mortality rate associated with the procedure is estimated at 0.2%.
F. Chen et al. (2021), in a meta-analysis of 74 articles (8,172 patients), noted a recurrence rate in 956 patients (11.6%). Factors contributing to a relatively higher recurrence rate included atypical symptoms of TN, absence of nerve excavation, non-arterial compression, patient age between 50-60 years, and longer disease duration. However, the recurrence rate after MVD was significantly lower than with pharmacotherapy, Gamma-Knife surgery, percutaneous balloon compression, and radiofrequency thermocoagulation. Even after successful surgery, 10% of patients experienced recurrences. The most common complications included hypoacusis (hearing loss) and TN paresis [37].
Postoperative hemorrhage into the cerebellum may be a major cause of fatal complications after MVD. If a hematoma is confirmed, urgent surgery should not be delayed. Rapid hematoma evacuation, as well as ventricular drainage through the anterior horns on both sides, can save the patient.
Stereotactic radiosurgery (SRS)
Lars Leksell (Fig. 12) first reported on stereotactic radiosurgery (SRS) in 1951. In his initial experiments, Leksell connected a dental X-ray tube to a stereotactic arc to irradiate the trigeminal ganglion. Later, in 1971, he developed the Gamma Knife, which uses multiple focused beams from cobalt-60 sources.

Fig. 12. Lars Leksell (1907–1986)
The first large series of 117 patients was conducted at the Mayo Clinic in Rochester, published by B. Pollock et al. (2002) with an average follow-up period of 26 months (ranging from 1 to 48 months). Pharmacotherapy was unnecessary for 57% of patients after one year, and 55% after three years. Sensory disturbances were detected in approximately 25% of patients at a dose of 90 Gy [38].
In the Marseille series of 497 patients with long-term follow-up, S. Tuleasca, J. Regis et al. (2016) reported that 64.9% and 45.3% of patients remained pain-free and did not require medication at five and ten years, respectively. Very troubling hypoesthesia was observed in only 3 patients (0.6%) [39].
The same authors, in 2018, performed a systematic review of all 65 published articles up to 2015, including 6,461 patients. Gamma - Knife was used in 45 articles, LINAC in 11, and CyberKnife in 9. The efficacy was found to be similar for each method: 53% for Gamma- Knife, 49% for LINAC, and 56% for CyberKnife. Recurrence rates ranged from 24% to 32%. Between 30% and 45% of patients remained pain-free without pharmacotherapy for up to 10 years of follow-up. The most common side effect was hypoesthesia (0–68%). Other side effects included dysesthesia, paresthesia, dry eyes, deafferentation pain, and keratitis [40].
According to a meta-analysis (46 articles, 5,787 patients) conducted by A. Gubian et al. (2017), the efficacy of SRS for classic TN is 71%, with over 64% of patients experiencing analgesic effects for five years or more after irradiation. Complications of SRS included temporary numbness and dysesthesia in 28% of patients, and hearing loss in 0.74%. TN Recurrence occurred in 25% of cases, but repeat irradiation remained effective [41].
There is now a large body of literature detailing the outcomes, particularly when using the Gamma-Knife system. A correlation has been established between the frequency of hypoesthesia and pain relief. Reoperation is possible even for the third time, although the incidence of hypoesthesia increases with each subsequent procedure. Overall results seem comparable to percutaneous methods but inferior to MVD. The likelihood of a favorable long-term outcome after Gamma-Knife surgery is slightly less than 50%, with a 20% chance of facial numbness.
A selective series of operations performed in leading clinics (Table 5).
Table 5. Selected large series of surgical treatment for trigeminal neuralgia
|
Procedure |
Author |
Years |
Total number of operations |
|
Balloon compression |
Yi Ma (China) [24] |
2000-2017 |
Exceeds 16 000 |
|
O. Barlas (Turkey) [23] |
2007-2021 |
500 |
|
|
Glycerol rhizotomy |
LZ Chen (China) [42] |
1983-2008 |
4012 |
|
XH Wang (China) [43] |
1983-2003 |
3370 |
|
|
D. Kondziolka (USA) [44] |
1985-2004 |
1174 |
|
|
Radiofrequency thermocoagulation |
Y. Kanpolat (Turkey) [18] |
1974-1999 |
2138 |
|
G. Nugent (USA) [45] |
1982-1997 |
Exceeds 1600 |
|
|
M. Sindou (France) [46] |
1980-2022 |
3250 |
|
|
Stereotactic radiosurgery |
C. Tuleasca (France) [39] |
1992-2010 |
497 |
|
K. Marshall USA) [47] |
1998-2008 |
777 |
|
|
A. Niranjan (USA) [48] |
1988-2016 |
1250 |
|
|
A. Jarrahi (USA) [49] |
2000-2022 |
587 |
|
|
Microvascular decompression |
P. Jannetta (USA) [35] |
1969-1999 |
4415 |
|
J. Zhong (China) [50] |
2000-2020 |
Exceeds 10 000 |
Algorithm for selecting surgical treatment for trigeminal neuralgia
Many universities and major centers in the USA, UK, Italy, South Korea, and other countries offer various algorithm options for selecting surgical interventions for TN. These algorithms are primarily designed for researchers, postgraduate students, and doctoral candidates, consisting of diagnostic and treatment blocks. For practicing physicians, in our opinion, a simpler algorithm would be more suitable (Fig. 13).
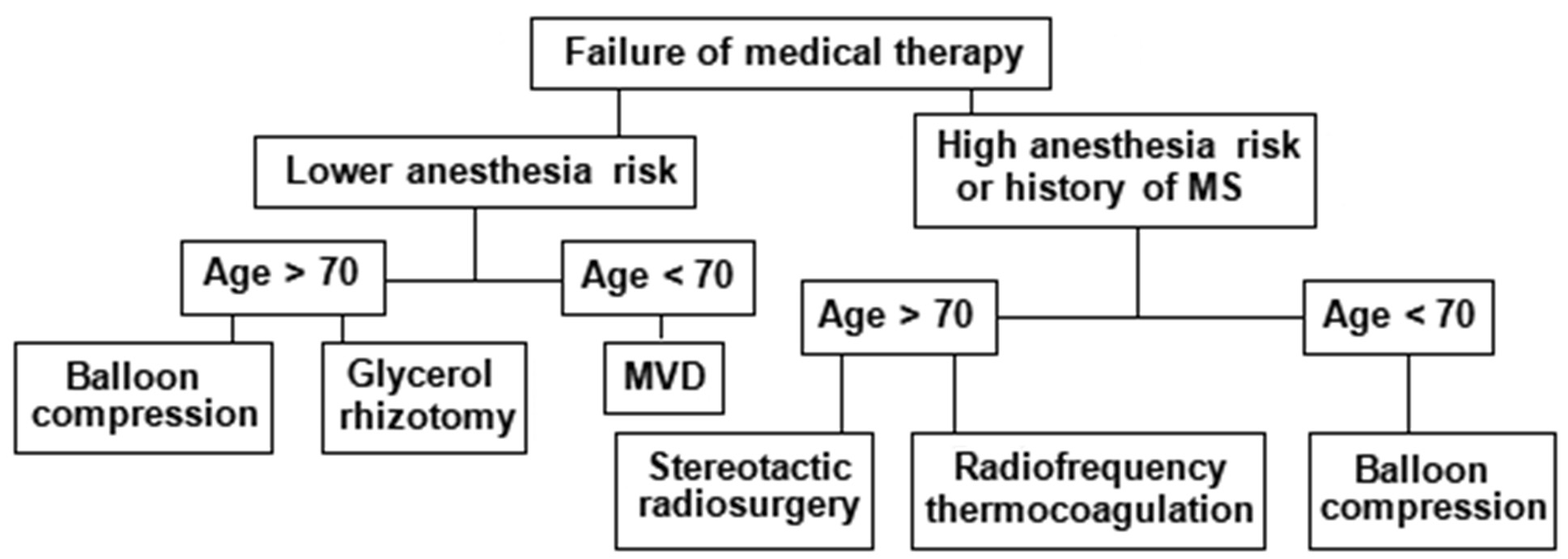
Fig. 13. An algorithm for refractory classical or idiopathic TN
1.For young and healthy patients without comorbidities, MVD should be offered.
2.For elderly, less healthy patients, or those who do not wish to undergo open surgery, balloon compression is recommended as the first-line treatment.
3. If balloon compression is not possible, glycerol rhizotomy or thermocoagulation may be considered, but thermocoagulation should be avoided in patients with pain in the first branch of the TN.
Selecting the most appropriate treatment method requires an assessment of the risks and benefits of each procedure. As always in neurosurgery, proper patient selection can ensure a higher success rate.
A summary table on the effectiveness of surgical treatments for TN (Table 6).
Table 6. Summary of surgical outcomes in TN (according to S. Bick et al. [51], R. Kirollos et al. [52] with modifications)
|
Procedure |
Initial Response Rate (%) |
Long-Term Response Rate (%) |
Recurrence Rate (%) |
Mortality (%) |
Side effect / Complications (%) |
|
MVD |
80.3-96.0 |
1 year - 84 |
23.4 |
0-1.2 (cerebellar infarct) |
Transient: trochlear palsy 0-5, cerebellar infarct 1, CSF leakage 2-17 |
|
RF |
97.6-99.0 |
1 year - 61.8 |
39.6 |
0.1 by carotid injury |
Transient: masseter weakness 4-24, keratitis 1-8, trochlear palsy 1-2 |
|
GR |
71.0-97.9 |
1 year - 53-63 |
61.5 |
0 |
Permanent: herpes eruption 50, meningeal reaction - frequent, numbness/dysesthesia 30, corneal hypoesthesia 15, keratitis 5 |
|
BC |
82.0-93.8 |
1 year - 74.6 |
33 |
0.2 by carotid injury |
Transient: numbness 39, masseter weakness 66, aseptic meningitis 0.7 |
|
SRS |
79.0-91.8 (delayed 10 d-3.4 mo) |
1 year - 75-90 |
46 |
0 |
Permanent: hypoesthesia 0-54 |
Medical negligence and lawsuits
A. Boyke et al. (2021) conducted a 34-year analysis of medical negligence lawsuits in the U.S. related to the treatment of TN. The report includes 49 lawsuits from 1985 to 2019. The most frequently accused medical professionals were dentists (31 cases) and neurosurgeons (10 cases). The average payout for dentists was $415,908, while for neurosurgeons it was $618,775. The most common complaints by plaintiffs after surgery involved cranial nerve injuries, loss of consortium, financial losses, and death [53].
Current clinical trials
As of December 8, 2022, there were 793 clinical trials registered on the website www.clinicaltrials.gov focusing on the treatment of neuropathic pain, of which 64 (7%) were specifically for TN [54].
Professor Joanna Zakrzewska from University College London (UCL) is, as of June 24, 2024, the lead moderator of fifteen new trials in the United Kingdom. For more information, [see https://www.hra.nhs.uk/planning-and-improving-research/application-summaries/research-summaries/?query=Trigeminal+Neuralgia&research_type=&rec_opinion=&date_from=&date_to=&relevance=true].
The most significant ones are:
1.Search for genes predisposing to TN - DNA will be collected from 500 patients with TN across multiple centers. A whole-genome analysis will be performed and compared with appropriate control standards to identify sequence variants that significantly segregate among TN patients. These sequence variants may cause TN by directly affecting the function of the proteins encoded by these genes or by altering other aspects of gene expression. If genetics indeed play a role in TN, there is a strong likelihood that this project will uncover these genes and, ultimately, the pathophysiological mechanisms involved.
2.Virtual Reality (VR) technology - this is being explored as a new tool to reduce pain perception and could become a breakthrough in cases resistant to treatment. A study will be conducted on the effectiveness of stereotactic radiosurgery with VR training. Additionally, using MRI and artificial intelligence, researchers will attempt to identify structural abnormalities in the central nervous system associated with pain.
3.Augmented Reality (AR) technology - combined with established trajectory planning software like Magic Leap 1 and Brainlab Elements, augmented reality will significantly improve the accuracy of foramen ovale puncture compared to the Härtel's "freehand" approach.
4.Artificial intelligence for predicting postoperative outcomes.
5.Biomarker research - this involves studying the expression levels of inflammatory cytokines and neurotransmitters in the peripheral blood of TN patients before and after surgery. The aim is to gather information for personalizing treatment options.
6.Percutaneous balloon compression using the Sinovation R neurosurgical robot.
Conclusions
- A cure for trigeminal neuralgia is unpredictable.
- Women experience recurrences more frequently than men, and the time to recurrence is shorter.
- Female gender and younger age are independently associated with worsening pain scores at the 3-month follow-up, according to multivariate analysis.
- In 10% of patients with classical TN who underwent successful surgery, recurrence is observed.
- A shorter disease duration (≤ 5 years), arterial compression, and Type I TN predict more favorable outcomes.
- In 74% of patients, microvascular decompression (MVD) results in pain-free status for up to 10 years, with the lowest rate of rehospitalization within the first year.
- While MVD carries a higher risk of serious complications, including weakness of the masticatory muscles, hearing loss, cerebellar infarction, and cerebrospinal fluid leakage, the rate of these complications remains relatively low in experienced hands.
- Among percutaneous techniques, radiofrequency thermocoagulation provides good initial and long-term pain relief, with the advantage of selecting the exposure to the specific branch of the trigeminal nerve.
- Glycerol rhizotomy and balloon compression have similar initial pain relief and duration of effect.
- Radiofrequency thermocoagulation is likely to require follow-up procedures.
- Advanced age and postoperative numbness are predictors of good - outcomes for percutaneous surgery.
- Repeated procedures carry a higher risk of pain recurrence, but the initial effectiveness of these subsequent procedures does not decrease.
- It is becoming increasingly clear that no single method has 100% efficacy. This suggests that TN is probably a heterogeneous group of disorders that collectively manifest as facial pain.
Destructive methods are preferred if MRI does not reveal neurovascular contact. The neurosurgical arsenal offers a wide range of options, each with its own advantages and disadvantages. Careful consideration of these factors will help determine which procedure is most suitable for a specific situation.
Currently, there is no ideal surgical method for treating TN that is minimally invasive, equally effective, and free from complications, failures, and recurrences. MVD remains the standard against which all other modern procedures are measured, offering the longest pain-free intervals.
Ultimately, uncovering the molecular mechanisms underlying trigeminal neuralgia will pave the way for new, more effective, and less invasive treatments.
Disclosure
Conflict of interest
The authors declare no conflicts of interest and no personal financial interest in the preparation of this article.
Funding
The study was conducted without sponsorship.
References