Original article
Ukrainian Neurosurgical Journal . 2024;30(3):18-29
https://doi.org10.25305/unj.301385
1 Romodanov Neurosurgery Institute, Kyiv, Ukraine
2 Pain Management Center SPRAVNO, Kyiv, Ukraine
3 Main Medical Center of the Ministry of Internal Affairs of Ukraine, Kyiv, Ukraine
Received: 04 April 2024
Accepted: 29 May 2024
Address for correspondence:
Dmytro M. Romanukha, Main Medical Center of the Ministry of Internal Affairs of Ukraine, 1 Berdychivsʹka Street, Kyiv, 04116, Ukraine, e-mail: neuromanukha@gmail.com
Patients with chronic abdominal pain are a complex cohort of patients who undergo treatment by many specialists for a long time: surgeons, urologists, gynecologists, neurologists, psychiatrists, etc. However, despite all diagnostic and treatment measures, the pain syndrome persists or worsens.
Objective ‒ evaluation of the effectiveness, safety and long-term results of treating patients with abdominal pain syndromes, which includes the use of various methods of minimally invasive interventions on the celiac plexus (CP) taking into account the peculiarities of the origin, nature and localization of pain.
Materials and methods: An analysis of the results of 26 interventions on CP in 21 patients was performed. Inclusion criteria for participants in the study were individuals with persistent pharmacoresistant abdominal pain for ≥3 months, aged 19 to 73 years. There were 13 (62.0%) male and 8 (38.0%) were female. Mean age was 55.2±15.2 years. Patients were divided into two groups. The first (n=16) included patients with pancreatic cancer, the second (n=5) included patients with non-oncological chronic abdominal pain syndromes: functional abdominal pain syndrome was diagnosed in three cases, and one observation each of solaritis and chronic pancreatitis.
All procedures were performed under CT. To assess the intensity of the pain syndrome, a visual analogue scale (VAS) of pain from 1 to 10 cm was used, where 0 cm is the absence of pain, 10 cm is unbearable pain; functional status (FS) - according to the Karnofsky scale (KS) from 0 to 100%. Estimation of the daily dose of opioid analgesics was estimated using the oral morphine equivalent daily dose (oMEDD). Patients were observed for 6 months, evaluations were carried out after 1 week, 1, 3 and 6 months, respectively.
Results: In the first group, 17 interventions on CP were performed in 16 participants, sympatholysis was performed twice in one patient. In the second group - 9 interventions in 5 patients: 4 Celiac Plexus Blocks (CPBs) of the central nervous system using "Depo-Medrol®" (methylprednisolone) and 5 neurolysis with 96% ethyl alcohol. Two patients were initially treated with CPB and then sympatholysis due to the recurrence of pain syndrome with the aim of a more stable sympatholytic and analgesic effect. In one patient, neurolysis of CP was performed three times. In all cases, no complications were recorded during the procedures.
VAS before the procedure in the general group (n=26) was 9.6±0.6 cm, one week after the intervention it was 4.5±1.6 cm (P<0.0001), after one month it was 3.2±1 .5 cm (P<0.0001), after 3 months – 3.0±1.6 cm (P<0.0001), after six months – 4.4±1.6 cm (P<0.0001). The FS indicator according to the KS before the procedure in the general group was 65.8±7.0%, one week after the intervention – 80.8±8.0% (P<0.0001), one month later – 81.5±8.3 % (P<0.0001), after 3 months – 75.0±9.5% (P<0.0010), after six months – 68.0±9.4% (P=0.4042). The oral morphine equivalent daily dose before the procedure in the general group was 123.8±86.0 mg per day, one week after the intervention on CP oMEDD was 57.3±61.2 mg (P<0.0001), after 1 month – 41.0±47.3 mg (P<0.0001), after 3 months – 44.0±51.3 mg (P<0.0001), after 6 months – 80.6±77.2 mg (P<0,0001).
Conclusions: Computed tomography-guided celiac plexus neurolysis is a useful and effective tool in treating patients with both abdominal pain caused by inoperable pancreatic cancer and chronic non-oncological pharmacoresistant abdominal pain. Minimally invasive interventions on CP provide a significant reduction of pain syndrome according to the VAS scale (p<0.001), reduce the need to take opioids analgesics (p<0.001) after 1, 3, 6 months and increase the FS of patients according to the KS (p<0.001) after 1, 3 months. Taking into account the high percentage of recurrence of pain syndrome in the studied patients of the group of non-oncology pain, the need for repeated interventions for the purpose of long-term pain control, interventions on CP in this cohort of patients require further research with an increase in the number of observations.
Key words: neurolysis; sympatholysis; celiac plexus; solar plexus; pancreatic cancer; abdominal pain; solaritis; functional abdominal pain syndrome
Introduction
According to the WHO, about 37% of people in developed countries suffer from diseases and conditions associated with chronic pain [1]. Studies conducted in Europe have shown that every fifth person reports the presence chronic pain of moderate or high intensity, with 90% of these individuals experiencing pain for more than two years, and one-third of cases not being alleviated by treatment [1]. Extrapolating these data suggests that millions of people in Ukraine, mainly of working age and elderly, have problems related to chronic pain.
Patients with chronic abdominal pain represent a challenging cohort, often undergoing long-term treatment by various specialists (surgeons, urologists, gynecologists, neurologists, psychiatrists, etc.). However, despite all diagnostic and therapeutic measures, the pain syndrome persists or worsens. A striking example is abdominal pain in patients with malignant neoplasms of the abdominal organs, as about half of cancer patients experience it, and in the later stages of the disease, this number exceeds 70% [2,3]. Its prevalence is even higher in patients with pancreatic cancer [4]. Only 12–20% of these patients are diagnosed at a stage where tumor resection is possible [5]. Pain management in these patients is usually very challenging, often requiring the chronic use of high doses of opioid and non-opioid analgesics or their combinations. Opioids are more effective and provide good pain relief but come with a range of side effects (nausea and vomiting, constipation, itching, dry mouth, pronounced sedation or delirium, hallucinogenic effects, the need to increase the dose due to the development of tolerance, intolerance to a particular drug) [6-8]. These side effects can worsen the quality of life, which is crucial for this cohort of patients, whose five-year survival rate is only 8% [9]. Inadequate pain management negatively affects the quality of life and is associated with worse clinical survival outcomes for patients [4, 10, 11].
The approach to treating patients with chronic non-oncological abdominal pain is a complex issue and is discussed in the literature. These pain syndromes are complex conditions diagnosed in a small number of patients. Often, various specialists examine the patients, but a definitive diagnosis cannot be established.
The functional abdominal pain syndrome (FAPS) is classified as a functional gastrointestinal disorder according to the Rome diagnostic criteria [12]. According to the latest revision (2016) of this diagnostic classification, FAPS is referred to as "centrally mediated abdominal syndrome." This can be a debilitating disorder characterized by constant or frequently recurring abdominal pain lasting at least six months, with some loss of daily functioning [13]. As with other functional gastrointestinal disorders, there is no evidence of structural (morphological) disease causing the symptoms.
Solar plexitis (solariitis) is rarely diagnosed in clinical practice. Patients typically describe it as pain primarily in the epigastric area between the xiphoid process of the sternum and the navel. The pain is cramp-like, not associated with food intake, and may radiate throughout the abdomen, under the ribs, and into the back. In addition to pain, the clinical picture may include spasms, atony of the stomach or intestines, abdominal distension, nausea, constipation, or diarrhea. Solar crises—episodes of intense, stabbing pain in the epigastrium—can also occur, manifesting as increased blood pressure, tachycardia, and skin redness. The etiology of solar plexitis is not well studied. It is presumed to involve infectious diseases, traumatic factors (abdominal or chest injuries), inflammatory diseases of the abdominal organs (particularly pancreatitis, cholecystitis), intoxications of various origins, etc.
The incidence of chronic pancreatitis is estimated to be 50–75 cases per 100,000 people per year [14]. About 85-90% of patients with this condition experience pain at the time of diagnosis, which worsens as the disease progresses, significantly impairing their quality of life [15]. Numerous studies on the quality of life of these patients indicate that pain dominates the quality of life indicators in all major areas [16,17]. Abdominal pain due to chronic pancreatitis is debilitating for the patient and poses a complex challenge for both gastroenterologists and pain management specialists (algologists) or surgeons.
Interventions on the structures of the autonomic nervous system are becoming increasingly common worldwide. They are safe, minimally invasive, effective, and associated with a minimal number of complications while providing a lasting therapeutic effect. However, only a small number of scientific studies have been published on minimally invasive interventions on sympathetic plexuses, particularly in cases of abdominal pain syndromes, and there is a lack of clear systematization of interventions and data on their outcomes and effectiveness.
It is important to understand the fundamental difference between CPB and neurolysis (sympatholysis) of nerve plexuses. CPB is performed with injectable corticosteroids and/or a combination of long-acting local anesthetics for a temporary block of pain impulse transmission. Neurolysis is performed using ethanol or phenol, which provides a more sustained effect by destroying nerve fibers. In some cases, radiofrequency denervation (ablation) or nerve plexus modulation is used for a longer-lasting effect.
Objective: To evaluate the effectiveness, safety, and long-term outcomes of treating patients with abdominal pain syndromes using various methods of minimally invasive interventions on the abdominal plexus, considering the nature, character, and location of the pain.
Materials and Methods
Study Design
A prospective interventional study was conducted on the basisof two medical institutions in Kyiv (Main Medical Center of the Ministry of Internal Affairs of Ukraine and Acad. A.P. Romodanov Institute of Neurosurgery of the National Academy of Medical Sciences of Ukraine) from 2016 to 2024. The results of 26 interventional procedures on the CP in 21 patients were analyzed. All procedures were performed using a standardized protocol by a single team consisting of three doctors (two from the hospital and one from the Institute).
The study was approved by the Ethics and Bioethics Committee of the A.P. Romodanov Institute of Neurosurgery of the National Academy of Medical Sciences of Ukraine (minutes No. 3, dated December 16, 2020). After a detailed explanation of the procedure, written informed consent was obtained from all patients. The study did not pose any increased risk to the participants and was conducted in compliance with bioethical norms and scientific standards for conducting clinical research involving patients.
Inclusion Criteria
Individuals with persistent pharmacoresistant abdominal pain lasting ≥3 months, diagnosed with pancreatic cancer, functional abdominal pain syndrome (FAPS), solaritis, or chronic pancreatitis. Patients of both sexes, aged 19 to 73 years. Lack of response to analgesics, anti-inflammatory drugs, and other treatments.
Exclusion Criteria
Age under 16 years. Patients with existing local infection at the puncture site or systemic infection (sepsis). Allergy to any anesthetic or contrast dye.
Individuals with impaired coagulation profile, presence of aneurysm, mural thrombus, or significant atherosclerotic calcification of the aorta. Patients with mental disorders under psychiatric observation. Inability to continue participation in the study throughout the observation period.
Group Characteristics
The study included 13 (62.0%) men and 8 (38.0%) women. The average age of the subjects was (55.2 ± 15.2) years. The patients were divided into two groups: the first group consisted of 16 patients with pancreatic cancer, and the second group included 5 patients with non-oncological chronic abdominal pain syndromes (three cases of FAPS, one case of solaritis, and one case of chronic pancreatitis).
Procedure Methodology
Patients were selected for empirical analysis of CP injections under computed tomography (CT) guidance, using a "GE Revolution Evo" 64/128-slice machine (General Electric, USA) at the Main Medical Center of the Ministry of Internal Affairs, and a "Toshiba Aquilion Prime" 80/160-slice machine (Toshiba, Japan) at the Institute of Neurosurgery. Prior to the procedure, all patients had their blood pressure, heart rate, and oxygen saturation levels measured. In cases of malignant pancreatic lesions, patients were cachectic, elderly, and had low blood pressure. An intravenous catheter was placed before the procedure due to the frequent complication of hypotension. For several days before the sympatholysis, all patients were advised to drink at least 1.5-2.0 liters of water per day; if this was not possible, intravenous infusion of 500-1000 ml of saline solution was administered. The neurolysis was performed under local anesthesia, with all patients undergoing cardiorespiratory monitoring (electrocardiography, blood pressure control, pulse oximetry) in the presence of an on-duty anesthesiologist during the procedure. There were no cases requiring intravenous sedation with fentanyl, midazolam, or general anesthesia with intubation.
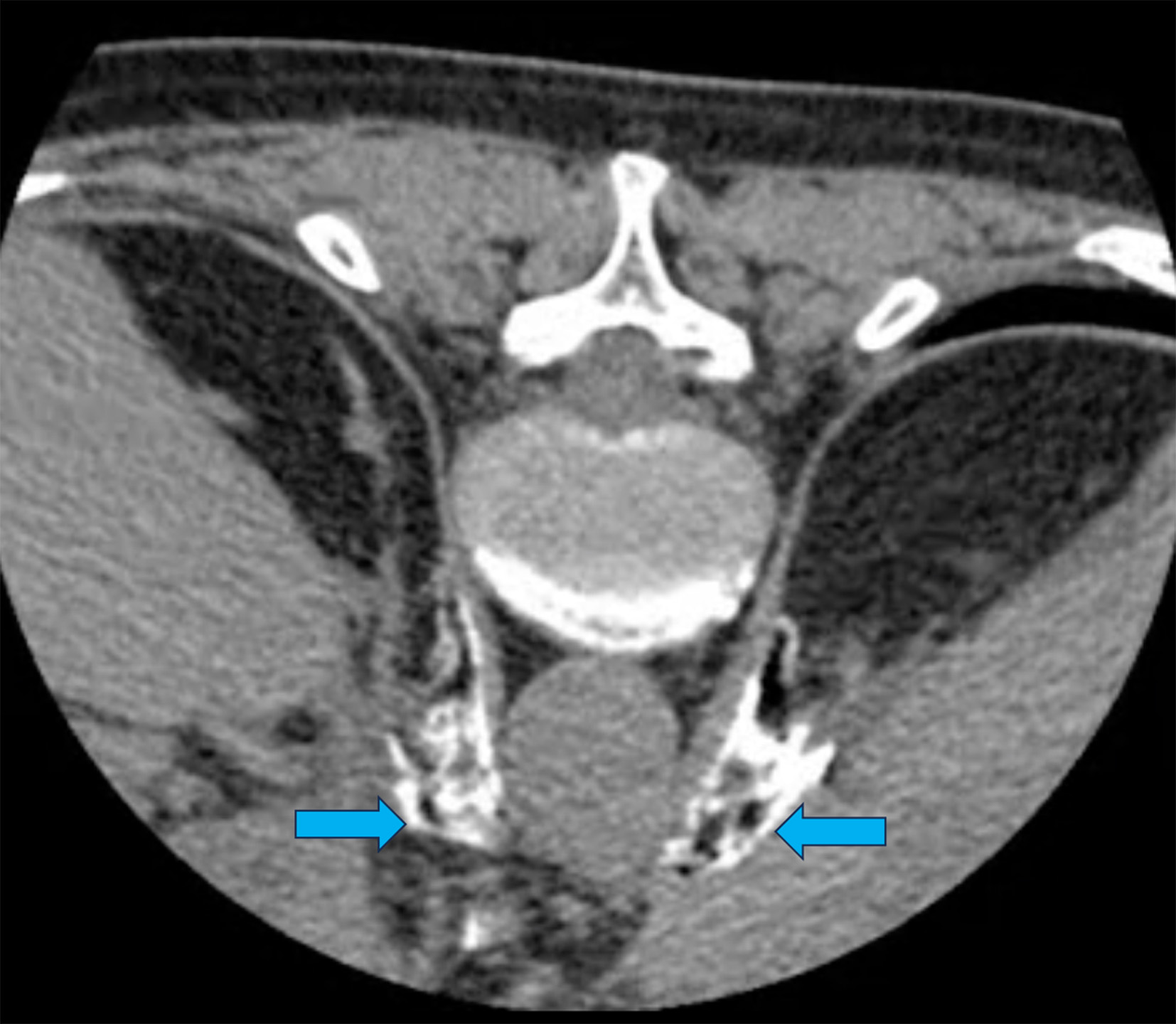
The optimal puncture site is located 5-7 cm lateral to the midline at the level of the L1 vertebra or at the level of the lower edge of the 12th rib, with the needle directed medially at a 45° angle and upwards (cranially) at a 15° angle. Following all aseptic rules, after subcutaneous infiltration with a 2% lidocaine solution, a 22G needle with a beveled tip, 120 mm in length, was gradually advanced forward alongside the vertebral bodies. The ideal position for the needle tip is approximately 1 cm anterior to the aorta, between the diaphragmatic crura and the pancreas, at the level between the celiac trunk and the superior mesenteric artery, as confirmed by control CT scanning. On each side, 1 ml of "Tomogexol 350" (Pharmak, Ukraine) radiopaque dye diluted in saline (1:2-1:3) was injected, followed by 5 ml of 0.5% bupivacaine to reduce pain response during alcohol infusion. Then, 20 ml of 96% ethyl alcohol was slowly injected (10 ml on each side). In cases of CPB "Depo-Medrol®" 40 mg (methylprednisolone, Pfizer, USA) was used instead of alcohol (40 mg on each side, totaling 80 mg). On CT imaging it is crucial during to confirm the spread of the neurolytic agent (or hormone) along the anterolateral surface and anterior to the aorta in the retroperitoneal space, as the spread of the neurolytic agent is key to successful sympatholysis (Fig. 1).

Fig. 1. Control CT Scan of a 64-year-old female patient with pancreatic cancer, undergoing celiac plexus neurolysis via bilateral posterior paravertebral antecrural access. The pre-procedure VAS pain score was 10 cm. The needles are positioned in the antecrural space at the level of the celiac artery trunk, with contrast injected to confirm their location. Arrows indicate the spread (free diffusion) of the contrast in the antecrural space along the lateral and anterior surface of the aorta and the celiac artery trunk—locations where the CP nodes are situated. Subsequently, 20 ml of 96% ethanol was injected. VAS score after the procedure: 2 cm
For analysis, data were collected from patients after the procedure at intervals of 1 week, 1 month, 3 months, and 6 months. Patients who were unable to visit the clinic were contacted by phone, and their responses were recorded. Data were also analyzed from patient examinations using a preliminary survey that used the Visual Analog Scale (VAS) for pain, ranging from 1 to 10 cm, where 0 cm indicates no pain, and 10 cm indicates unbearable pain. Before and after the procedure, the functional status (FS) of patients was assessed using the Karnofsky scale (KS), which ranges from 0 to 100%. The daily dose of opioid analgesics was evaluated using the oral morphine equivalent daily dose (oMEDD). Participants were followed-up for 6 months, with assessments conducted at 1 week, 1 month, 3 months, and 6 months.
Statistical Analysis
The obtained data were processed using the MedCalc V 22016 statistical software package. Quantitative data (age, VAS score, and KS) are presented as the arithmetic mean and standard deviation. To identify differences after the interventions, the Student's t-test for related samples was used if the data followed a normal distribution, or a Wilcoxon rank sum test was used if the data distribution deviated from normal. A critical significance level of 0.05 was adopted.
Results and Discussion
The total number of CP interventions was 26 in 21 patients. In most cases (76.2%), the pain was caused by malignant pancreatic lesions (Table 1).
Table 1. Characteristics of patients (n=21)
|
Indicator |
Number |
||
|
Abs. |
% |
||
|
Sex |
|
|
|
|
Males |
13 |
62,0 |
|
|
Females |
8 |
38,0 |
|
|
Age, years: |
|
||
|
mean |
55,2±15,2 |
||
|
min-max |
19‒73 |
||
|
Etiology of pain: |
|
|
|
|
Pancreatic cancer |
16 |
76,2 |
|
|
FAPS |
3 |
14,2 |
|
|
Solaritis |
1 |
4,8 |
|
|
Chronic pancreatitis |
1 |
4,8 |
|
No complications were recorded during the interventions that would lead to a deterioration of the patients' condition. There were no manifestations of neurological deficits, vascular injuries, perforations of internal organs, pleural sinuses, lungs, etc.
In 5 cases (19.2%), local pain at the injection site was reported, which regressed within 24 hours (Table 2). This complication is associated with the spread of residual ethanol from the needle into the surrounding tissues (subcutaneous tissue, muscles) at the puncture site. To prevent this complication, we recommend injecting 3-5 ml of saline before removing the needles. In 4 cases (15.4%), post-procedural pain was noted in the lower abdomen, radiating along the ureter to the lower back and groin area. In all cases, the complications regressed within 24 hours after the intervention. A possible explanation for this pain syndrome is the injury to the renal capsule as the needle passes near it or irritation of the capsule by ethanol. Two patients (7.7%) reported transient diarrhea after the procedure. This complication is likely due to the activation of the parasympathetic nervous system's influence on the gastrointestinal tract as a result of blocking the fibers of the CP. The diarrhea was transient and did not require specific treatment. In 1 case (3.8%), orthostatic post-procedural hypotension was recorded, which was resolved by intravenous administration of crystalloids and dexamethasone. This complication is caused by sympathetic denervation (CPB) of the vascular wall of the large arteries of the abdominal aorta. The patient's blood pressure normalized within a few hours of observation.
Table 2. Adverse events and post-procedural complications of celiac plexus interventions (n=26)
|
Indicator |
Number |
|
|
Abs. |
% |
|
|
Post-Procedural Complications: |
|
|
|
no complications |
14 |
53,9 |
|
local pain at the puncture site |
5 |
19,2 |
|
pain along the ureter |
4 |
15,4 |
|
transient diarrhea |
2 |
7,7 |
|
orthostatic hypotension |
1 |
3,8 |
In the overall patient group, a significant reduction in pain according to the VAS was recorded one week after the procedure: from (9.6 ± 0.6) before the intervention to (4.5 ± 1.6) cm (Table 3). A persistent (up to 6 months) and noticeable decrease in pain intensity on the VAS was noted before and after the intervention (p < 0.001) (Fig. 2).
Table 3. Changes of VAS and KS scores (n=26)
|
Assessment period |
VAS score, sm |
P |
KS score, % |
P |
|
Before procedure |
9,6±0,6 |
<0,001 |
65,8±7,0 |
<0,001 |
|
After 1 week |
4,5±1,6 |
<0,001 |
80,8±8,0 |
<0,001 |
|
After 1 month |
3,2±1,5 |
<0,001 |
81,5±8,3 |
<0,001 |
|
After 3 months |
3,0±1,6 |
<0,001 |
75,0±9,5 |
<0,001 |
|
After 6 months |
4,4±1,6 |
<0,001 |
68,0±9,4 |
0,4042 |
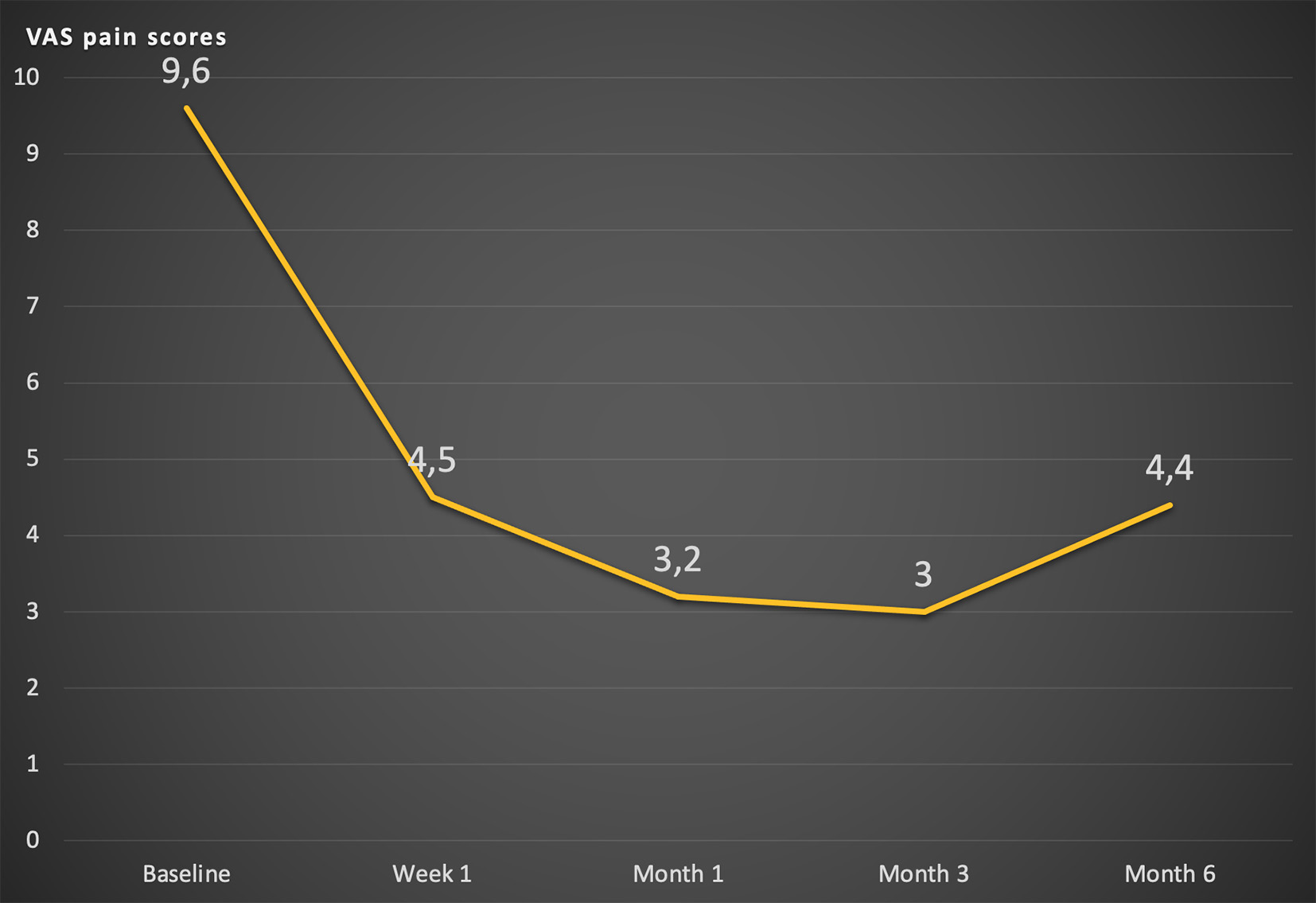
Fig. 2. Changes of average VAS value
The average KS score before and one week after the procedure was (65.8 ± 7.0)% and (80.8 ± 8.0)%, respectively, with this indicator increasing in the first three months following the procedure (the difference was statistically significant). The decrease in functional status at six months is related to the predominance of oncology patients in the study and the complications associated with the primary disease.
The daily dose of opioid analgesics in the general group before the procedure was (123.8 ± 86.0) mg, one week after the CP intervention, it was (57.3 ± 61.2) mg (p < 0.0001), one month after the procedure – (41.0 ± 47.3) mg (p < 0.0001), three months after – (44.0 ± 51.3) mg (p < 0.0001), and six months after – (80.6 ± 77.2) mg (p < 0.0001) (Fig. 3).
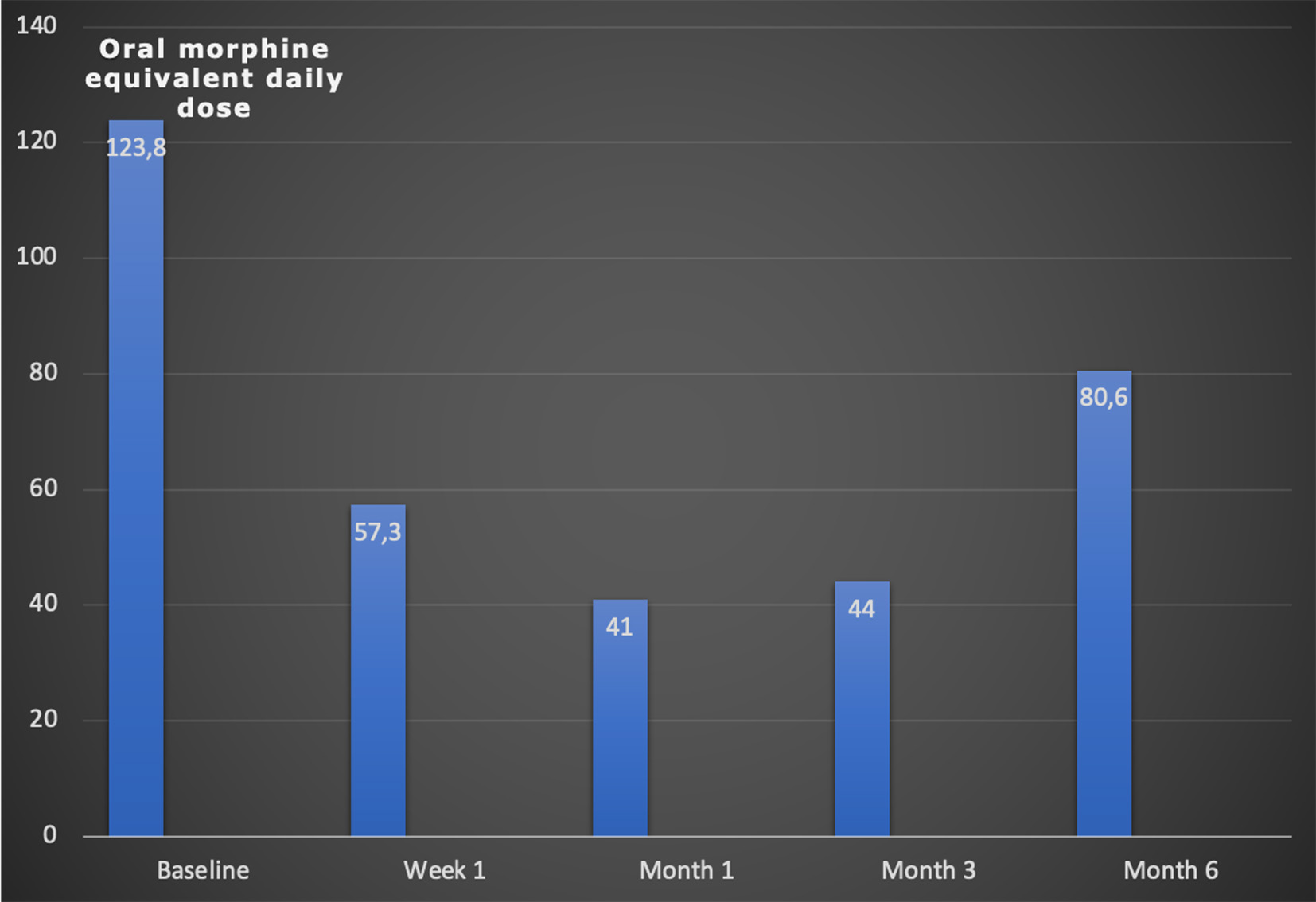
Fig. 3. Changes of the average oMEDD
In the overall group, a repeat CP intervention was performed in 3 (14.0%) patients. In 1 (4.8%) patient, the CP neurolysis was performed three times. In the group of patients with malignant pancreatic tumors, CP neurolysis was repeated only in 1 (6.25%) case.
In all cases of malignant pancreatic lesions, neurolysis of the CP fibers with ethanol was used. In the second group, four CPBs using "Depo-Medrol®" (methylprednisolone) and five neurolyses with 96% ethyl alcohol were performed.
The average VAS score before the procedure in the first group was (9.7±0.6) cm, one week after the intervention it was (4.7±1.4) cm (p<0.0001), one month after it was (3.1±1.5) cm (p<0.0001), three months after it was (2.6±1.3) cm (p<0.0001), and six months after it was (4.1±1.4) cm (p<0.0001), the FS indicator according to the Karnofsky scale (KS) score before the procedure was (64.7±7.9)%, one week after the intervention it was (78.2±6.4)% (p<0.0001), one month after it was (78.8±6.9)% (p<0.0001), three months after it was (71.2±6.9)% (p=0.023), and six months after it was (63.5±6.0)% (p=0.668). The oMEDD before the procedure was (179.0±43.8) mg, which can be explained by the high level of opioid analgesic use in cancer patients. After the intervention, this figure decreased to (85.0±58.6) mg (p<0.0001); one month and three months later, it was (61.8±46.5) mg and (66.5±50.6) mg, respectively (p<0.0001). However, after six months, the need for opioids in this cohort of patients increased again to (120.6±66.0) mg/day (p=0.025), although it remained lower than the pre-procedure level.
All patients in the second group experienced significant pain reduction according to the VAS, both one week after – from (9.4±0.5) cm before the procedure to (4.0±1.9) cm (p<0.001) – and six months after – to (4.8±1.8) cm (p<0.001). The average KS score one week after the procedure increased from (67.7±4.4)% to (86.6±8.6)% (p<0.007), and six months later to (77.7±8.3)% (p=0.017). The use of opioid analgesics was significantly lower in this group. The oMEDD before the procedure averaged (20.0±26.0) mg, after the procedure – (5.0±10.6) mg (p<0.001), and this level remained stable for six months after the intervention.
The literature mainly includes single observations of CP interventions, case series, or small patient samples. Despite the significant interest of specialists in such interventions, there are challenges in finding and selecting patients for this procedure. Healthcare professionals and patients are not well informed about this treatment approach. A meta-analysis of 24 studies resulted in 59 publications after a literature search, with only 24 providing data on CP interventions in two or more patients [18]. In the review by S. Vig et al., a literature search yielded 686 publications, but only 44 randomized controlled trials and case series (more than 10 patients) were selected for analysis [19]. The limitations of our study include the small sample size of patients with non-oncological pain who underwent celiac plexus interventions, which can be attributed to the complex diagnosis of such abdominal pain syndromes and the selection of patients suitable for this procedure.
CP neurolysis demonstrates high efficacy in reducing pain intensity, decreasing the need for opioid medications, and minimizing associated side effects in patients with malignant abdominal organ lesions. This has been confirmed in numerous studies [8,9,20–24]. A Cochrane review involving six studies showed statistically significant evidence of the benefits of CP over conservative pain management in all patients who underwent the procedure [25]. According to S. Vig et al., CP interventions have proven to be an effective method for treating pain in malignant pancreatic lesions [19].
The pathophysiology of FAPS is unique because the pain is almost entirely caused by enhanced central perception of normal visceral signals, rather than increased peripheral stimulation from the abdominal or pelvic organs. This clinical feature often arises when gastrointestinal disorders become chronic, and the perceived pain (in the cortical centers) increasingly depends on the input from the central nervous system, which is modulated by psychosocial variables. In fact, with FAPS, gastrointestinal disorders may be minor or even absent, resulting in "abnormal perception of normal bowel function." Thus, while the pain is felt in the abdomen (and attributed to it), the nature and magnitude of the pain are predominantly regulated by cognitive and emotional centers. Recognizing this concept is crucial for understanding FAPS from the perspective of clinical manifestations, pathophysiology, diagnosis, and treatment [26-28].
According to the Rome IV criteria, FAPS must include all of the following [26]:
These criteria should be observed for the past 3 months with symptom onset at least 6 months before the diagnosis.
If the diagnostic criteria of FAPS are as specified, further diagnostic testing is not required. Unfortunately, most patients undergo extensive testing, including non-invasive procedures such as abdominal ultrasound, multislice CT, and magnetic resonance imaging (MRI) of the abdomen, as well as invasive procedures such as capsule video endoscopy, esophagogastroduodenoscopy, colonoscopy, endoscopic ultrasonography (EUS) and retrograde cholangiopancreatography etc. These tests are not only unnecessary but also pose a risk to the patient, lead to excessive healthcare costs, and can reinforce the patient's inclination to believe that a different diagnosis has not been established. Along with the lack of experience and confidence in the diagnosis on the part of the physician, this often leads to prolonged testing [27].
Thus, if the diagnostic criteria are met and there are no alarming signs, a diagnosis of FAPS can be established if there is no suspicion that the pain is feigned. Feigned pain or malingering is associated with the deliberate fabrication or gross exaggeration of physical (or psychological) symptoms, driven by external incentives. Malingering is not easy to detect, especially for physicians inexperienced with this pathology, so it may be advisable to consult a psychiatrist to confirm or refute the suspicion [27,29].
Medication therapy may be targeted at symptoms or underlying causes, that is, the central mechanisms of pain. Due to persistent debilitating abdominal pain, many patients require and receive analgesic medications, often opioids. Many overburdened emergency department physicians, faced with demanding patients without a clear cause of their pain, prescribe them [30]. Besides the obvious problems and side effects associated with excessive use of opioid analgesics, there is a less recognized potential complication: the development of opioid bowel syndrome. This syndrome is characterized by chronic or frequently recurring abdominal pain that worsens with prolonged use or increasing doses of opioids [31]. Since it presents the same symptom as FAPS, the relationship between pain and opioid use is unclear. Some researchers believe that the cause lies in paradoxical visceral hyperalgesia induced by chronic opioid use [30,32].
The basis of medical therapy for FAPS is treatment with antidepressants [27]. This is because these drugs can modulate pain perception by modulating central regulatory mechanisms and, to some extent, visceral hypersensitivity. They are successfully used to treat chronic neuropathic pain [33]. A systematic review and meta-analysis of tricyclic antidepressants, selective serotonin reuptake inhibitors, serotonin-norepinephrine reuptake inhibitors, and psychological therapy (cognitive behavioral therapy) have shown that all these treatments were effective [33].
The main issues related to antidepressant therapy for FAPS are side effects and the perception of many patients that being prescribed a "psychiatric" drug means that all their problems are "in their head." For this reason, it is important to articulate the reasons for prescribing these medications in such a way as to persuade the patient to try them while simultaneously reducing the frequency of side effects [27,28,34].
One possible treatment method for FAPS is minimally invasive intervention on the nerve structures of the sympathetic system [35,36]. However, there is a limited amount of literature on such interventions for chronic functional abdominal pain. Successful use of radiofrequency ablation of the thoracic splanchnic nerves has been reported in a 27-year-old man who had previously undergone a diagnostic CPB with lidocaine, which showed significant pain reduction on the Visual Analog Scale (VAS). During the 8-week follow-up period, the patient was satisfied with the result and reported that his pain had decreased [37]. In a study that performed 72 minimally invasive interventions on the CP or splanchnic nerves, 5 were conducted in patients with chronic abdominal pain syndrome. A 20% (1/5) effectiveness of such interventions was reported [38].
In the International Classification of Diseases X Revision, the term "solaritis" is not present. In the Rome IV diagnostic criteria, there is no mention of a diagnosis of solaritis. In the section on functional gastrointestinal disorders, epigastric pain syndrome is described with the following diagnostic criteria [26]: it should include one or both of the following symptoms occurring at least 1 day per week:
1) bothersome pain in the epigastrium (severe enough to interfere with usual activities);
2) unpleasant burning in the epigastrium (severe enough to interfere with usual activities);
absence of signs of organic, systemic, or metabolic disease that is likely to explain the symptoms during routine investigations (e.g., fibrogastroduodenoscopy).
These criteria are considered valid if they have been observed during the last 3 months, with symptom onset at least 6 months prior to diagnosis.
Supporting criteria:
In contemporary medical literature, the term "solaritis" is scarcely mentioned. There is no data on the prevalence of this condition. Older French literature sources discuss pain arising from irritation of the fibers of the celiac nerve plexus, referred to as "solar neuralgia," "solaralgia," or "solaritis" [39]. However, due to the poorly understood etiological factors of this disease and the difficulty in diagnosing it, interest in pain originating in the solar plexus has significantly diminished. One theory for the development of solaritis was Glénard's visceroptosis, which involves the prolapse of abdominal organs, leading to the exposure of the aorta located directly beneath the abdominal wall. Tension in the intestinal mesentery irritates the nerve plexuses, causing pain as a result.
Maratka examined a consecutive series of 234 patients admitted to the hospital regardless of diagnosis and 100 outpatient patients with established gastrointestinal clinics with functional disorders. The overall prevalence of a certain degree of solar tenderness in unselected patients was 28%, and 37% in patients with functional gastrointestinal disorders [40]. The author uses the term "solar tenderness" to describe pain upon palpation of the aorta and believes that, normally, the periaortic nerve plexuses are painless upon palpation. In some patients, such palpation is painful, sometimes very painful, so the author suggests considering "solar tenderness" as a pathology. "Solar tenderness" has a typical characteristic that distinguishes it from other causes of tenderness upon palpation in the epigastrium. Sensitivity is localized along the course of the aorta along the midline between the xiphoid process and the navel and bifurcates below the navel into two branches, corresponding to an inverted Y-type bifurcation. This is best detected by using the fingertips of both hands placed side by side perpendicular to the abdominal wall. Moving the hands from one side of the midline to the other, one can find that the tenderness is strictly limited to the aorta and its bifurcation. Moving the hands distally shows that the tenderness is greatest above the navel and disappears when the hands are positioned between the branches of the bifurcation. Tenderness limited to the aorta and its branches indicates that the origin of the pain is from the periaortic nerve plexuses, as there is no other organ or structure with such localized tenderness. In more complex cases, in addition to objective "solar tenderness," there is a subjective component—"solar pain," spontaneous pain arising in the solar plexus. It is felt in the epigastrium and radiates laterally along both costal margins, as well as to the back. It is often associated with a sensation of pressure and tightness in the epigastrium. A typical complaint is an unpleasant pulsation of the aorta ("epigastric pulsation"). The pain is not related to food intake, is sometimes associated with stress, rarely occurs at night, and may be accompanied by anxiety and other symptoms of neurosis. According to the author, "solar tenderness" and "solar pain" form a typical syndrome—the solar plexus syndrome, which is a specific type of neurosis characterized predominantly by the involvement of abdominal nerve plexuses, often associated with functional gastrointestinal disorders such as functional dyspepsia and irritable bowel syndrome [40].
According to Z. Maratka, the Rome criteria incorrectly interpret some pathological conditions and fail to mention typical syndromes, particularly pain resulting from solaritis [41,42]. Our patient was clinically diagnosed with "solaritis" due to the combination of pain syndrome with symptoms like abdominal bloating and constipation.
Treating pancreatic pain can be challenging and often ineffective [43]. A variety of approaches are used to manage these patients, including pancreatic enzymes, octreotide, antioxidants, opioid analgesia, minimally invasive celiac plexus interventions, and endoscopic pancreatic surgery. Endoscopic treatment involves the removal of stones from the main pancreatic duct and dilation of strictures, which facilitates decompression and drainage of the main pancreatic duct [44]. According to one popular hypothesis, the pain is caused by duct obstruction. The expected result of duct obstruction is increased pressure within the duct and gland, especially when pancreatic secretion is stimulated (e.g., after eating). Elevated pressure can lead to pain due to increased basolateral enzymes secretion or ischemia in the pancreas resulting from pressure rising to a level that impedes its blood supply [45]. Studies conducted on both animals and humans have shown that patients with chronic pancreatitis may have increased ductal or parenchymal pressure in the pancreas. Surgical treatment is associated with a reduction in this pressure and a decrease in pain intensity [46,47]. Animal models of chronic pancreatitis also show that pancreatic blood flow decreases and ischemia develops upon stimulation of pancreatic secretion, mimicking compartment syndrome. While this mechanism of pain onset seems plausible, other studies have not found a clear relationship between pressure and pain or between pressure reduction and pain relief [47]. Extracorporeal shock wave lithotripsy of pancreatic stones reduces obstruction of the main pancreatic duct, leading to pain relief [48]. Other surgical methods include thoracoscopic splanchnicectomy (denervation of the visceral nerves, nerve plexuses, and ganglia), intraoperative CPB, radiofrequency ablation or cryoablation, and pancreatic resection [15]. However, these treatments have had varying and limited efficacy for long-term pain control. Some patients respond to combined multimodal therapy, but many patients become dependent on opioids. For this reason, the use of opioids to treat chronic pain raises resistance and concerns about potential opioid dependence and other side effects of opioids [49].
The ultimate common pathway for any pathological process causing pain in the pancreas is the visceral nerves [50]. The sympathetic nerves that innervate the pancreas pass through the CP, and blocking this innervation can be highly effective in treating pancreatic pain [7].
Celiac plexus interventions are an alternative method for reducing pain syndrome in patients with pharmacoresistant chronic pancreatitis [51-54]. M. Kaufman et al. conducted a thorough search of the Medline, Pubmed, and Embase databases for studies published in the English literature from January 1966 to December 2007 that evaluated the effectiveness of ultrasound-guided CPB in patients with chronic pancreatitis whose conservative medication therapy failed to control the pain and improve their condition [7]. Studies involving fewer than 10 patients were excluded from the analysis. Six relevant studies were identified (total number of patients = 221). It was found that ultrasound-guided CPB block was an effective tool for reducing abdominal pain in 51.46% of patients.
In a prospective randomized study on the efficacy of ultrasound-guided CPB in treating abdominal pain associated with chronic pancreatitis, conducted by F. Gress et al. [55], the procedure was performed on 90 individuals (40 men and 50 women). Significant pain reduction was reported in 55% of patients. The average pain score on the VAS decreased from 8 to 2 cm at 4 and 8 weeks post-procedure (p<0.05). A sustained effect was noted at 12 weeks in 26% of patients and at 24 weeks in 10%. In 3 patients, pain was absent at 35 and 48 weeks of follow-up. According to the authors, the procedure is less effective in patients who have undergone surgery for chronic pancreatitis.
M.S. Sey et al. studied the efficacy of endoscopic ultrasound-guided (EUS) interventions on the CP in 1,108 patients treated at the Indiana University Medical Center (USA). A total of 248 patients with chronic pancreatitis, who underwent two or more CP interventions, were selected. After the first procedure, 76% of patients reported a reduction in pain intensity. The average duration of pain relief was 10 weeks. Subsequent procedures contributed to a prolonged period of pain relief (12–20 weeks). Older age (p = 0.026) and pain reduction after the first block (p = 0.0024) were associated with pain reduction following subsequent procedures. Considering the near absence of complications (3 minor transient complications), the study by M.S. Sey et al. demonstrates the feasibility and effectiveness of repeated celiac plexus interventions for pain control in patients with chronic pancreatitis [56].
Various minimally invasive intervention techniques targeting structures of the sympathetic autonomic nervous system (SANS) for treating pain caused by chronic pancreatitis are compared. For instance, D. Santosh et al. compared CP using a percutaneous technique under fluoroscopic control of an image intensifier (fluoroscopy) and under endoscopic ultrasound guidance [57]. The study involved 56 patients with chronic pancreatitis and abdominal pain requiring daily analgesic intake for 4 weeks or more. CPB was performed in 27 patients using EUS, and in 29 patients using fluoroscopy. Improvement in pain score on the VAS was observed in 70% of individuals who underwent the EUS-guided technique and in 30% of the percutaneous access group (p=0.044).
A case involved the use of botulinum toxin for CP in a 32-year-old patient with pharmacoresistant chronic pancreatitis who continued to experience pain even after surgical treatment, such as pancreatic duct drainage [58]. This treatment option for persistent chronic pancreatic pain was considered because clinical studies on botulinum toxin have shown good efficacy in treating peripheral and central neuropathic pain, particularly in cases of postherpetic neuralgia, trigeminal neuralgia, and neuropathic pain caused by spinal cord injury. After the injection of 100 units of onabotulinumtoxin A into the CP under X-ray guidance, the pain disappeared, the patient did not request any opioid medications, and was discharged. A sustained result was demonstrated for 15 weeks following the intervention. The patient reported that pain intensity was 0 cm on the VAS without the use of analgesics.
As with the use of CP neurolysis for pain caused by pancreatic cancer, some researchers recommend performing CPB in the early stages of pain management for pancreatitis, especially before the patient becomes dependent on opioid medications [60]. Most authors believe that in cases of chronic pancreatitis, CP interventions should be limited to situations where pain does not respond to other treatments (medical and surgical) or for managing exacerbations of chronic pain [61, 62].
The current (2023) American Gastroenterological Association guidelines for endoscopic treatment of acute and recurrent chronic pancreatitis recommend CP interventions for patients with debilitating pain that significantly worsens their quality of life and for whom other therapeutic measures have been ineffective [6]. The rationale is based on observational studies suggesting that pain relief lasting about 6 months can be achieved in 50-60% of patients [7, 54, 64].
Conclusions
Disclosure
Conflict of Interest
The authors declare no conflict of interest.
Ethical Standards
All procedures performed on patients in the study comply with the ethical standards of the institutional and national ethics committees and the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
The study was approved by the Ethics and Bioethics Committee of the Romodanov Institute of Neurosurgery of the National Academy of Medical Sciences of Ukraine (minutes No. 3, dated December 16, 2020).
Informed Consent
Informed consent was obtained from all patients.
Funding
The study did not receive any sponsorship support.
References